Figures & data
FIGURE 1. ERp57, the calnexin/calreticulin cycle and PrP biosynthesis. (A) ERp57/PDIA3/Grp58 and PDIA1 are members of the Protein Disulfide Isomerase (PDI) family. The domain structure is conserved in other members of the PDI family and is indicated using color code: in blue, catalytic domains a and a’, in red and green, the non-catalytic b and b’ domains, respectively and in orange, the ER retention signal. (B) Schematic representation of the protein folding activity of ERp57, comprising the oxidation, reduction and/or the isomerization of disulfide bonds in nascent glycoproteins. (C) Schematic representations of the biosynthesis of Prion protein (PrP) through the secretory pathway (left panel), emphasizing the impact of ERp57 deficiency (right panel). Left panel: Translation of PrP is directed toward the lumen of the endoplasmic reticulum (ER), where it receives a series of post-translational modifications, including glycosylation, intramolecular disulfide bond, and a GPI anchor that targets the protein to the plasma membrane. During its passing through the ER, PrP undergoes processing and quality control, to then mature at the Golgi apparatus for its sorting to the plasma membrane. Around 10% of the newly synthesized PrP is not correctly folded, and undergoes clearance through the ER-associated degradation (ERAD) pathway. Right panel: ERp57 deficiency alters PrP biosynthesis leading to alteration in the ratio of glycosylation, accumulation of large aggregates of the protein due to cross-links by aberrant intermolecular disulfide bonds, and reduction of steady-state levels of PrP protein related to decreased half-life of the protein, which lead to altered PrP trafficking.
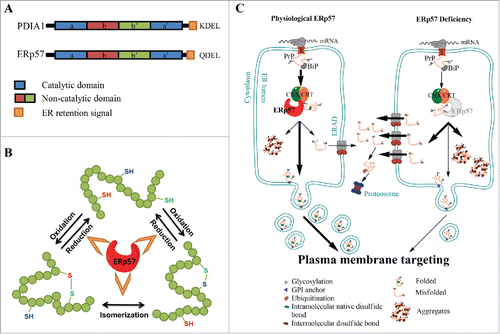
TABLE 1. Role of Protein Disulfide Isomerases in neurodegenerative diseases.
