Figures & data
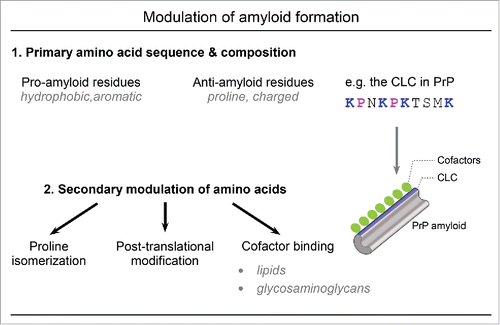
FIGURE 1. Amyloid formation can be influenced by both amino acid composition and secondary modulation of these amino acid residues. 1) Primary amino acid composition and sequence can influence the propensity for amyloid formation with hydrophobic and aromatic residues promoting amyloid formation and proline and charged residues impeding amyloid formation. The central lysine cluster (CLC) of mammalian PrP includes four lysine and 2 proline residues that prevent PrP amyloid formation. 2) Amino acids can be further modulated by proline isomerization, post-translational modifications, or cofactor binding to alter the amyloid propensity conferred by the primary amino acid sequence. In the case of PrP, polyanionic lipids or glycosaminoglycans (pictured in green) could bind to the CLC (in blue) to neutralize the anti-amyloid effects of the basic CLC lysine residues and promote PrP amyloid formation.