Figures & data
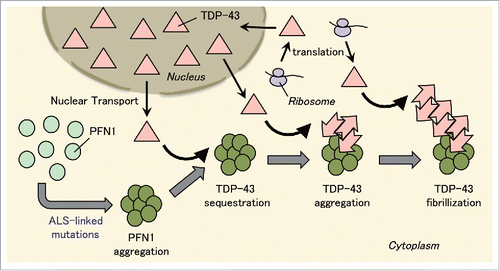
FIGURE 1. Conversion of normal TDP-43 into prion-like species by ALS-linked PFN1 mutants. PFN1 with mutations that cause ALS forms aggregates in the cytoplasm. TDP-43 localized in the cytoplasm for its production or transportation from nucleus is sequestered into PFN1 aggregates, where it is converted into prion-like species, and forms TDP-43 aggregates. Furthermore, these TDP-43 aggregates serve as a template for conversion of normal TDP-43 into prion-like TDP-43 aggregates. As a result, TDP-43 filaments are formed. Thus, TDP-43 aggregation is facilitated firstly by sequestration of TDP-43 into PFN1 aggregates and secondly by self-template-type aggregation of prion-like TDP-43.