Figures & data
FIGURE 1. Tau fibrils are internalized in non-neuronal and neuronal cells. A: Representative picture of HeLa (top panel) and CAD (bottom panel) cells respectively incubated with fluorescent Alexa-555 or 488 (red and green) recombinant tau fibrils for 16 hours and stained for the cytosol (in blue) and plasma membrane (WGA, green and red, respectively). Left panels: Merge of WGA and Tau; Right panels: single channel showing fluorescent Tau. Scale bar: 15 and 10 µm for HeLa and CAD respectively. B: Representative deconvolved pictures of HeLa (left, labeled with green WGA to delineate the plasma membrane) and CAD cells (right, labeled with red WGA to delineate the plasma membrane and TNTs) containing fluorescent tau fibrils (red and green, respectively for HeLa and CAD) and connected by TNTs containing several tau fibrils puncta (inset). Scale bar is 15 and 10 µm for HeLa and CAD respectively. Boxed insets show close-ups of the TNTs containing punctate Tau aggregates.
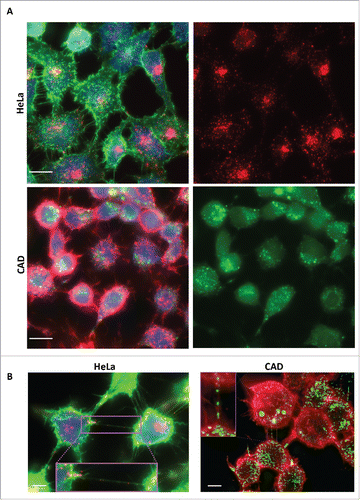
FIGURE 2. TNT number is increased in cells containing tau fibrils. A: Representative pictures of HeLa (top) and CAD (bottom) cells labeled with WGA-Alexa 488 (green) in control untreated conditions (left) or incubated with Alexa-555 tau fibrils (red) for 16 h (right). Scale bar: 15 and 10 µm for HeLa and CAD respectively. B: Quantification of TNT connected cells from experiment done in A. Absolute and relative data are shown in left and right respectively.
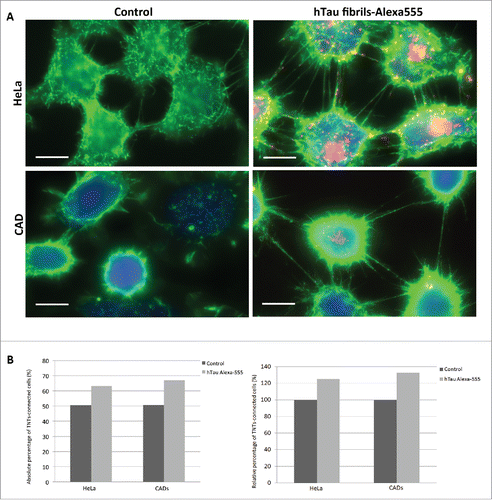
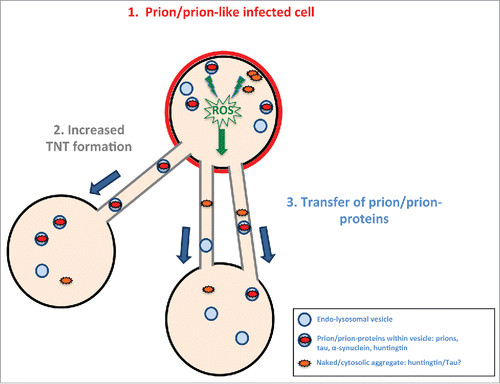
FIGURE 3. Model of prion/prion-like protein induced formation of TNTs and spreading. Prions or prion-like proteins such as tau fibrils after “infecting” a cell (in red) (1) induce via an unknown (possible common) mechanism (for example, induced via radical oxygen species (ROS) from oxidative stress) an increase in TNT number (2). The prion/prion-like aggregates would then be propagated via TNT-mediated intercellular trafficking from infected cells to naïve cells (3).