Figures & data
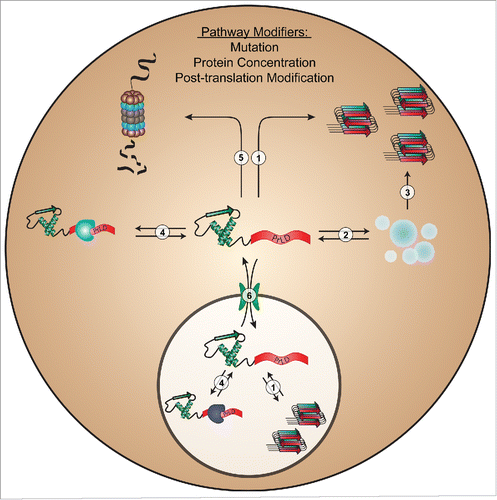
FIGURE 1. Intracellular processes commonly affecting PrLD aggregation. Mutations, changes in protein concentration, or post-translational modifications in PrLDs can affect protein aggregation by altering intrinsic aggregation propensity (1); liquid-liquid phase separation/stress granule dynamics (2); conversion of granules to a pathological state (3); organism-, tissue-, or compartment-specific intermolecular interactions (4); proteasome-mediated degradation (5); or nucleocytoplasmic transport (6).