Figures & data
FIGURE 1. Vaccine Production. (A) DNA was isolated from HEK293 cells infected with hAd5 and hAd5:tgG-RL and subjected to PacI digestion to identify the presence of tgG-RL coding sequence. (B) Western blot of conditioned media from either mock infected or hAd5:tgG-RL infected HEK293 cells. Proteins were resolved by SDS-PAGE, blotted to nitrocellulose and probed with either anti-tgG or anti-RL sera at 1:1000 or 1:2000, respectively. Protein-antibody complexes were probed with alkaline phosphatase labeled secondary antibody, and visualized following development with BCIP/NBT.
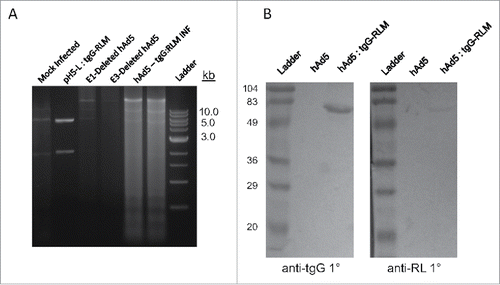
FIGURE 2. Systemic and Mucosal Epitope-specific Antibody Responses. White-tailed deer received an oral administration of 2.0 × 1010 viral particles of either hAd5 (n = 5) or hAd5:tgG-RL (n = 4). The animal who failed to mount an antibody response to the hAd5:tgG-RL vaccine was excluded from this consideration. Animals were orally immunized twice with a two-week interval. Serum antibody titers (A) and fecal antibody titers (B) were quantified with a capture ELISA using RL peptide to coat the wells. Data presented are as the mean ± 1 SD.
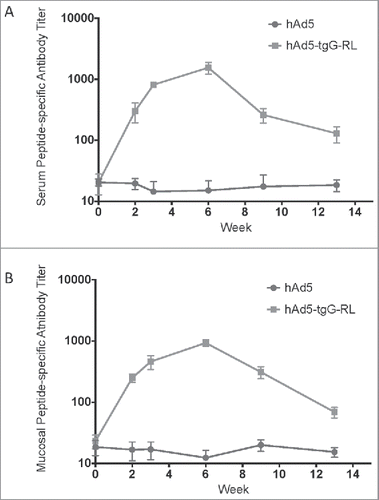
FIGURE 3. Lymphocyte Proliferation Assays. tgG-specific lymphocyte proliferative responses were determined following oral immunization of white-tailed deer with hAd5:tgG-RL vector. Lymphocytes were isolated from spleens 91 days post-immunization. Proliferative capacity of lymphocytes was determined by incorporation of [3H] thymidine follow co-culture with affinity purified tgG protein. Proliferative responses are reported as stimulation index (mean counts per minute with tgG stimulation of triplicate cultures /mean counts per minute of triplicate cultures with medium alone).
![FIGURE 3. Lymphocyte Proliferation Assays. tgG-specific lymphocyte proliferative responses were determined following oral immunization of white-tailed deer with hAd5:tgG-RL vector. Lymphocytes were isolated from spleens 91 days post-immunization. Proliferative capacity of lymphocytes was determined by incorporation of [3H] thymidine follow co-culture with affinity purified tgG protein. Proliferative responses are reported as stimulation index (mean counts per minute with tgG stimulation of triplicate cultures /mean counts per minute of triplicate cultures with medium alone).](/cms/asset/658cf79c-a645-4cf2-9945-121c07bec269/kprn_a_1367083_f0003_b.gif)
FIGURE 4. Specificity of Immune Responses. (A) Antibody titers were quantified by capture ELISA using cervid PrPC 90–231 as coating antigen, and are reported as mean values ± 1 SD. Monoclonal antibodies 6D11 and M2188 served as positive controls, while polyOv.RL sera served as a negative control. (B) Immunoprocipitation of PrP from healthy and prion-infected brains. Pooled serum from deer orally immunized with hAd5:tgG-RL was assessed for reactivity with PrPSc and PrPC. Serum antibodies were cross-linked to magnetic beads and incubated with non-infected and infected 10% brain homogenate. A PrPC/Sc reactive monoclonal antibody, AH6B, as well as polyclonal antibodies affinity-purified from serum samples collected following parenteral vaccination with a YML construct were included as controls. The YML antibody has demonstrated reactivity with PrPSc in immunoprecipitation assays.Citation37
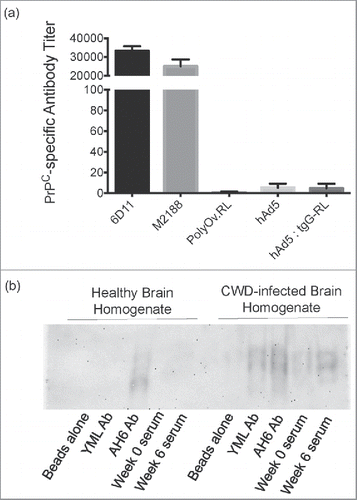
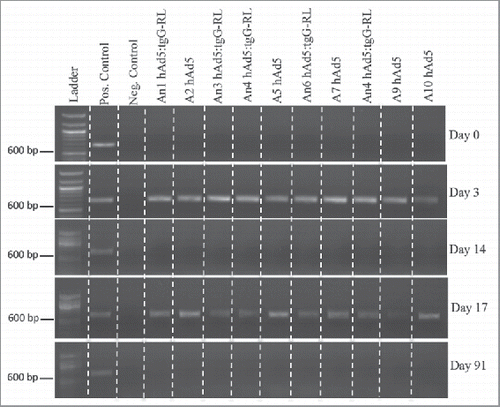
FIGURE 5. Vector Shedding. Ten white-tailed deer (n = 5/group) orally received 2.0 × 1010 viral particles of either hAd5 or hAd5:tgG-RL. Animals were orally immunized at D0 and D14. Total DNA was isolated from fecal samples on individual animals, and served as template for diagnostic PCR to identify viral sequence coding for the Ad5 hexon protein. DNA concentration was standardized for each 20 ul PCR, and entire reactions were resolved in 1% agarose gels.