Figures & data
FIGURE 1. Protein aggregates coalesce in daughter buds but not in mother cells. A. Sup35PrD-GFP was overexpressed for approximately 18 hours, and then placed on 8-well glass bottom slides and imaged for an additional 12 hours according to Sharma et al. (2017).Citation14 Of the 1382 cells that were imaged, 20.8% contained ring or line aggregates. Of these cells with aggregates, the initial formation of early foci was captured in 22 G2/M phase cells that contained early foci in both mother cell and daughter bud. These cells were followed until larger aggregates were assembled. Representative images of early foci initially appearing in both mother cell and daughter bud (01:00 minute) to their fusion in the daughter bud (04:00 minute) are shown. Yellow and green arrows point to the early foci in the mother cell, while the red and blue arrows are in the daughter bud, turning grey when they merge. B. Early foci trajectories (from part A) followed for 16 minutes are shown. The movement of each foci was tracked on a 2D plane, with colors corresponding to arrows in part A in the mother (left cell) and daughter bud (right cell). C. 22 time-lapse videos were analyzed for the number of aggregates in the mother cell and daughter bud present at the end of recordings, most of which ranged from one to six hours. The box-plot represents the mean (middle line), upper and lower quartiles and outliers (open circle). Statistically significant differences in aggregate formation in the mother cell and daughter bud were determined by a paired two-tailed t-test (p<0.0001).
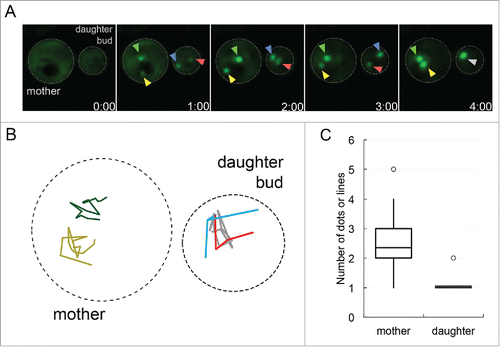
FIGURE 2. Endogenous Sup35p sediments in heavy fractions by 24 hours of induction. Sup35PrD-GFP was overexpressed for 8, 16, and 24 hours. Cultures were lysed using glass beads and protein was immediately separated by sucrose gradient centrifugation (20-60% sucrose). Samples were centrifuged at 4°C for 1 hour. Seven fractions were collected and run on SDS-PAGE for immunoblotting. Anti-Sup35C antibody was used in immunoblot analysis. An uninduced 24 hour culture, [PSI+] and [psi−] culture were also used as controls.
![FIGURE 2. Endogenous Sup35p sediments in heavy fractions by 24 hours of induction. Sup35PrD-GFP was overexpressed for 8, 16, and 24 hours. Cultures were lysed using glass beads and protein was immediately separated by sucrose gradient centrifugation (20-60% sucrose). Samples were centrifuged at 4°C for 1 hour. Seven fractions were collected and run on SDS-PAGE for immunoblotting. Anti-Sup35C antibody was used in immunoblot analysis. An uninduced 24 hour culture, [PSI+] and [psi−] culture were also used as controls.](/cms/asset/472afd9c-8bc5-461b-ace3-643ab2ebb2fe/kprn_a_1368606_f0002_b.gif)
