Figures & data
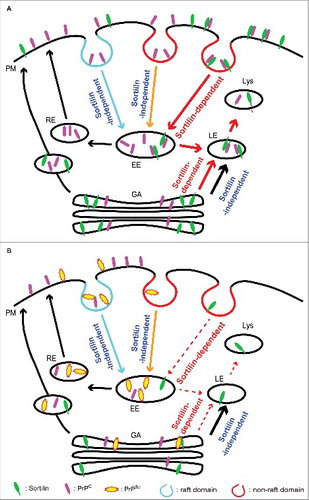
FIGURE 1. A model of the sortilin-mediated intracellular trafficking of PrPC and PrPSc in prion-uninfected and infected neurons. (A) Sortilin-dependent and -independent endocytosis of PrPC in uninfected neurons. Sortilin mediates endocytosis of PrPC on the plasma membrane (PM), particularly at non-raft domains, via the clathrin-dependent pathway to early endosomes (EE) and then traffics it to late endosomes/lysosomes (LE/Lys) for degradation. Other PrPC molecules are trafficked either to LE/Lys for degradation or to the recycling endosome (RE) pathway in a sortilin-independent way. There also might be sortilin-dependent and -independent trafficking pathways from the Golgi apparatus (GA) to LE/Lys for degradation. (B) Intracellular trafficking of PrPC and PrPSc in prion-infected neurons. Prion infection stimulates lysosomal degradation of sortilin via an unknown mechanism, thereby impairing the sortilin-mediated trafficking of PrPC and PrPSc to LE/Lys for degradation. As a result, PrPC and PrPSc are increased at raft domains and endocytosed via the sortilin-independent pathway to RE, causing accumulation of PrPSc and increasing conversion of PrPC into PrPSc in prion-infected neurons. PrPSc could undergo retrograde transport to the GA. However, sortilin might also be functionally impaired in the GA, thereby being unable to traffic PrPSc in the GA to LE/Lys for degradation. The decreased degradation of PrPSc in LE/Lys and the increased conversion of PrPC into PrPSc in raft domains or RE could both contribute to the constitutive production of PrPSc in prion-infected neurons. Dashed arrows indicate restricted trafficking.