Figures & data
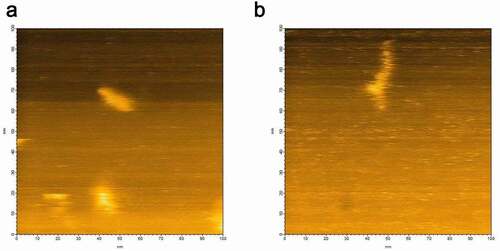
Figure 1. (a) HS-AFM image of hPrP showing various heterogeneous oligomeric states distributed from monomers to hexamers. (b) Typical hexameric oligomer of hPrP with a tail
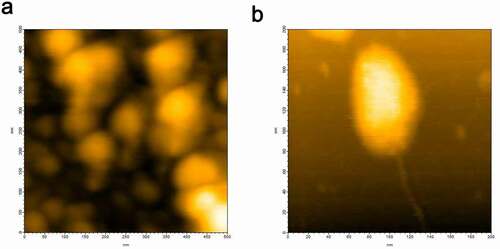
Figure 3. (a) HS-AFM image of hPrP and αS complex at equilibrium. (b) Height analysis showed the oligomer at equilibrium was a dimer
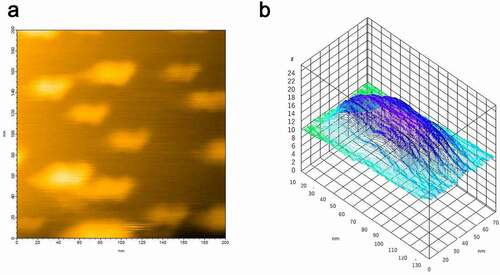
Figure 4. Dynamic light scattering (DLS) measurement of a number (population) of particles as a function of particle size (diameter) in (a) hPrP solution with peak at 24 nm, corresponding to an oligomer, (b) αS solution with a diameter of 1.7 nm, corresponding to a monomer, and (c) a mixture of hPrP and αS with a peak at the diameter of 1.7 nm corresponding to a hetero-dimer of hPrP and αS
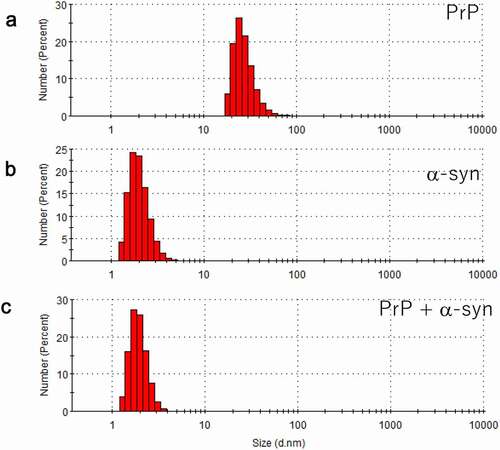
Figure 5. (a) [Citation1H-Citation15]N HSQC spectra of 200 µM [Citation15]N labelled hPrP without (blue) or with 220 µM non-labelled αS (red) at pH 6.1 in 99% H2O/1% D2O. (b) [Citation1H-Citation15]N HSQC spectra of 206 µM [Citation15]N labelled αS without (blue) or with 173 µM non-labelled hPrP (red) at pH 4.6 in 99% H2O/1% D2O
![Figure 5. (a) [Citation1H-Citation15]N HSQC spectra of 200 µM [Citation15]N labelled hPrP without (blue) or with 220 µM non-labelled αS (red) at pH 6.1 in 99% H2O/1% D2O. (b) [Citation1H-Citation15]N HSQC spectra of 206 µM [Citation15]N labelled αS without (blue) or with 173 µM non-labelled hPrP (red) at pH 4.6 in 99% H2O/1% D2O](/cms/asset/ef59581b-cb01-4c5f-8477-268e63a34618/kprn_a_1910176_f0005_oc.jpg)
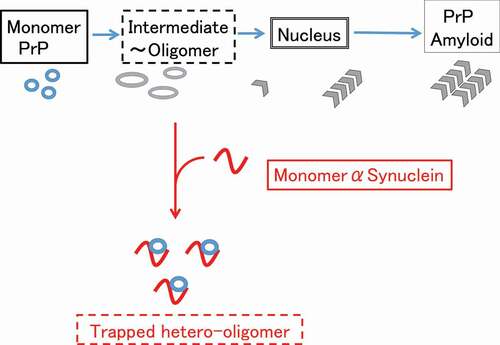
Figure 6. Possible mechanism of suppression of the pathogenic conversion of hPrP by αS. Monomeric αS can trap the intermediate state hPrP and forms a stable hetero-dimer, depleting the available monomeric hPrP for pathogenic conversion