Figures & data
Figure 1. Proposed mechanism of disease due to altered aEVO and its buffer βS in OLs. aEVO of APs, including αS and Aβ, might be an epigenetic phenomenon transmitted transgenerationally to confer resistance against the stressors in OLs in offspring during reproduction. By virtue of information carried by the amyloid protofibrils in reproduction, offspring can better cope with forthcoming stressors in the brain to reduce MS risk. However, increased aEVO in OLs might lead to MSA through the antagonistic pleiotropy mechanism during parental ageing. βS might act as a buffer for aEVO in reproduction that is beneficial in evolution. βS might be altered through the antagonistic pleiotropy mechanism in ageing, resulting in increased AP aggregation and OL degeneration, leading to MSA. Thus, it is predicted that reduced βS expression might increase αS evolvability in OLs, which is therapeutically beneficial for MS. On the other hand, a reduction in altered βS expression during ageing might be therapeutic for MSA.
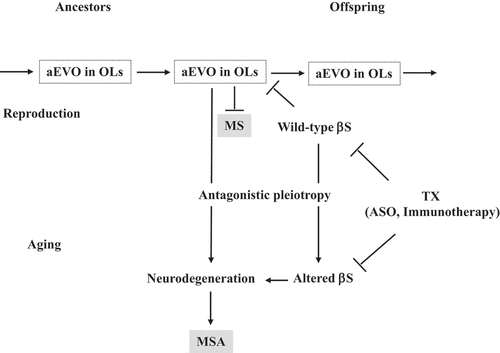
Figure 2. Clinical evidence supporting altered αS evolvability in disorders with abnormal OL function. (a), Levels of CSF αS in MS patients (n = 37) and in patients with clinically isolated syndrome (CIS, n = 36) were significantly decreased compared with those in benign neurological conditions (BNC, n = 38). **p< 0.01, ***p< 0.001. (b), Levels of αS and oligomeric αS in serum measured by ELISA were significantly lower in MS patients (n = 60) compared to the healthy control group (n = 60). ***p< 0.001. (c), Box-whisker plots revealed that oligomeric phosphorylated αS levels were elevated in post-mortem CSF derived from MSA (n = 8) and distinguished MSA from the other α-synucleinopathies, including PD (n = 38), DLB (n = 15), PSP (n = 12) and controls (n = 16), ***p< 0.001. (d), Detection of αS oligomers by immunoblotting. The sarkosyl-insoluble fractions of brain sample homogenates were analysed, and αS oligomers (asterisks) were visualized in fractions from MSA, PD and control (Ctrl) patients’ brain samples. F: frontal cortex, P: parietal cortex, T: temporal cortex. β-actin: loading control. Reprinted from Antonelou et al [Citation21]. (a), Bilge et al [Citation24]. (b), Foulds et al [Citation22]. (c) and Sekiya et al [Citation30]. (d) with permission.
![Figure 2. Clinical evidence supporting altered αS evolvability in disorders with abnormal OL function. (a), Levels of CSF αS in MS patients (n = 37) and in patients with clinically isolated syndrome (CIS, n = 36) were significantly decreased compared with those in benign neurological conditions (BNC, n = 38). **p< 0.01, ***p< 0.001. (b), Levels of αS and oligomeric αS in serum measured by ELISA were significantly lower in MS patients (n = 60) compared to the healthy control group (n = 60). ***p< 0.001. (c), Box-whisker plots revealed that oligomeric phosphorylated αS levels were elevated in post-mortem CSF derived from MSA (n = 8) and distinguished MSA from the other α-synucleinopathies, including PD (n = 38), DLB (n = 15), PSP (n = 12) and controls (n = 16), ***p< 0.001. (d), Detection of αS oligomers by immunoblotting. The sarkosyl-insoluble fractions of brain sample homogenates were analysed, and αS oligomers (asterisks) were visualized in fractions from MSA, PD and control (Ctrl) patients’ brain samples. F: frontal cortex, P: parietal cortex, T: temporal cortex. β-actin: loading control. Reprinted from Antonelou et al [Citation21]. (a), Bilge et al [Citation24]. (b), Foulds et al [Citation22]. (c) and Sekiya et al [Citation30]. (d) with permission.](/cms/asset/4da7c190-0945-433b-93d2-06149217f9b9/kprn_a_2172912_f0002_b.gif)
