Figures & data
Figure 1. Organization of the developing mouse neocortex. The two classes of neural precursor, radial glia and short neural precursors, both reside in the Ventricular Zone (VZ) with a process attached to the apical (luminal) surface adjacent to the developing lateral ventricle. The overt structural distinction between the 2 neural precursors is that radial glia possess a process attached to the basal (cortical) surface while short neural precursors do not. During mitosis the cell body migrates to the apical surface where it will divide to generate 2 daughter cells. In the case of radial glia, the daughter cells can be new radial glia cells, basal progenitors, or post-mitotic neurons. Basal progenitors are a transient type of progenitor that resides basal to the VZ in the Subventricular Zone (SVZ), and can also self-renew as well as generate post-mitotic neurons. Post-mitotic neurons migrate away from the VZ and/or SVZ along radial glia processes through the Intermediate Zone (IZ) to populate the developing Cortical Plate (CP) in an inside-out fashion, where the deepest cortical layers are generated first. The other apical precursor type, short neural precursors, can also self-renew or generate post-mitotic neurons, however they do not produce basal progenitors.
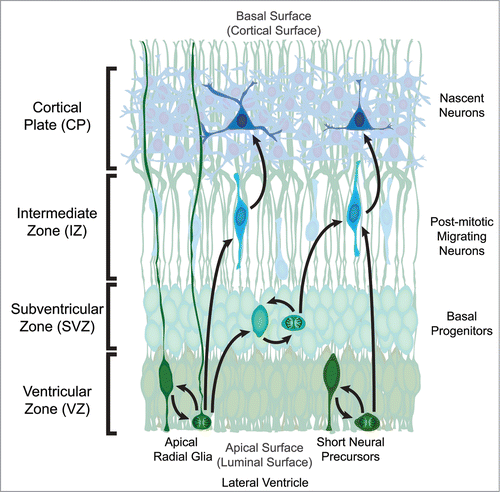
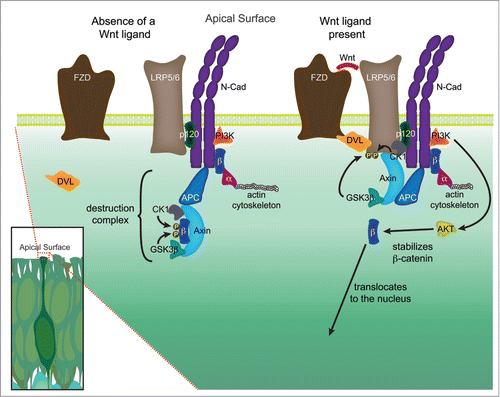
Figure 2. Hypothetical signaling hub, or extra-nuclear hub, containing both adherens junction and Wnt signaling components at the apical surface. The high density of adherens junction and Wnt signaling components located in the apical end feet of neural precursors located in the VZ, coupled with emerging data suggests that these pathways may physically and functionally interact. In the absence of a Wnt signal, a destruction complex comprised of Axin and APC bind to β-catenin (β) and recruit the kinases GSK3β and CK1, which phosphorylate β-catenin thereby targeting it for ubiquitination and ultimately destruction. In the presence of a Wnt ligand, a Frizzled (Fzd) and LRP5/6 complex is formed and bound by Dishevelled (Dvl), leading to the phosphorylation of the LRP5/6 receptor by several kinases, including GSK3β and CK1. The phosphorylated LRP5/6 receptor recruits axin to the plasma membrane, leading to the decay of the destruction complex, and allowing stabilized β-catenin to be translocated to the nucleus. A growing number of studies suggest that several key components of Wnt signaling may physically interact with members of the adherens junction, in particular N-cadherin (N-cad). N-cadherin binds both p120-catenin (p120) at the JMD domain (juxtamembrane intracellular domain) and β-catenin at the CBD domain (catenin binding domain). β-catenin in turn binds to α-catenin (α), which is dynamically tethered to the actin cytoskeleton. PI3K, which through activation of AKT stabilizes β-catenin allowing it to participate in signaling, has also been shown to interact with N-cadherin. The research detailing additional potential interactions are detailed in the main text.