Figures & data
Figure 1. Whole genome expression survey of the inflammatory response of HCAEC to Wnt5A and IL-1β. GO pathway maps of the 10 most significant processes regulated by (A) Wnt5A (250 ng/mL) and (B) IL-1β (20 U/mL). Genes are clustered according to biological processes. Pathways represented as histograms are ranked by the –log value (P value). Length of histogram corresponds to the number of genes associated with that specific pathway.
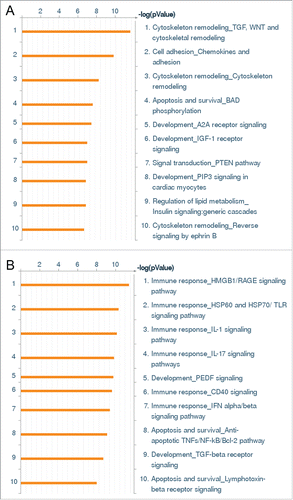
Table 1. Genes regulated by both Wnt5A and IL-1β in “Cytoskeleton remodeling_TGF, WNT and cytoskeletal remodeling” and “Cell adhesion_Chemokines and adhesion” pathways.
Table 2. Genes regulated only by Wnt5A in “Cytoskeleton remodeling_TGF, WNT and cytoskeletal remodeling” and “Cell adhesion_Chemokines and adhesion” pathways.
Table 3. Genes regulated by IL-1β in “Immune response_HMGB1/RAGE signaling pathway” and “Immune response_HSP60 and HSP70/TLR signaling pathway.”
Figure 2. Expression of Fzd5 and Ryk receptors in HCAEC. Expression of mRNA levels of (A) Fzd5 and (B) Ryk in HCAEC treated with Wnt5A and IL-1β (IL-1b) for 8 h as outlined in Methods. Data were obtained from 3 independent qRT-PCR experiments with duplicate samples and expressed as the mean ± SEM. **P < 0.01, IL-1β versus untreated. (C) Quantitative ratio of constitutive levels of Fzd5 and Ryk mRNAs in HCAEC. ***P < 0.001, Ryk vs. Fzd5 by Student's t-test. (D) Protein expression of Fzd5 and Ryk receptors in HCAEC either untreated (left panel) or treated with IL-1β (IL-1) for 24 h. Images show immunofluorescence staining using specific antibodies for Fzd5 and Ryk (red), F-actin (phalloidin, green) and nuclei (DAPI, blue). Zeiss Axioskope, original magnification 200x.
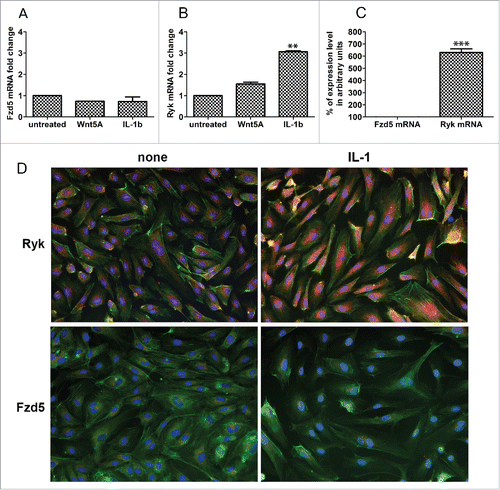
Figure 3. Effects of ROCK inhibition on Wnt5A-induced phosphorylation of LIMK2 and CFL1. Immunofluorescence staining of (A) phosphorylated LIMK2 and (B) phosphorylated CFL1 (both in red) and nuclei (blue) in HCAEC treated with either Wnt5A alone or in combination with the ROCK inhibitor Y-27632 (iROCK) for 1 h and 4 h respectively. Zeiss Axioskope, original magnification 200x. (C) Mean cellular fluorescent intensities of phosphorylated LIMK2 and CFL1 quantified by ImageJ based Fiji software. pLIMK2, phosphorylated LIMK2; pCF1, phosphorylated CFL1. ***P < 0.001, Wnt5A versus none and Wnt5A combined with iROCK vs. Wnt5A alone.
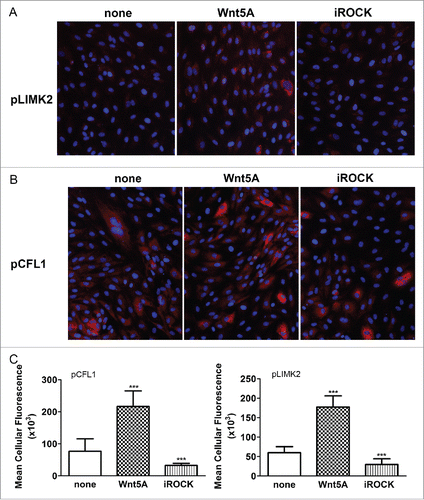
Figure 4. Actin stress fiber formation and adherens junction disruption in HCAEC in response to Wnt5A and IL-1β. (A) Actin stress fibers stained by live actin-RFP in HCAEC either untreated (left panel) or treated with Wnt5A (middle panel) or IL-1β (IL-1, right panel), in the absence or presence of sFRP1 or WIF. Stained cells were captured at 8 h after treatment using a Zeiss Axio Observer.Z1, original magnification 400x. (B) Immunofluorescence staining for β-catenin and VE-cadherin (red) in HCAEC treated with either Wnt5A or IL-1β (IL-1) for 8 h. Nuclei are stained blue. Arrowheads point to inter-endothelial gaps. Zeiss Axioskope, original magnification 400x.
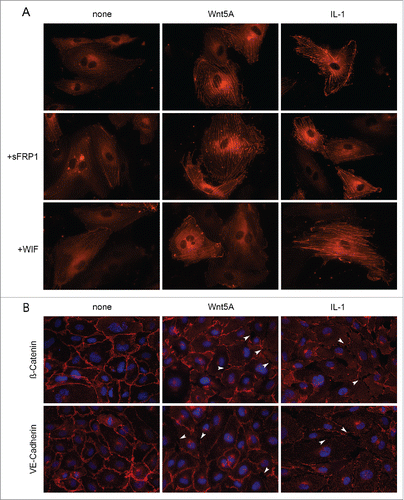
Figure 5. ECIS barrier function assays in HCAEC. Uniform confluent monolayers of HCAEC cultured in stabilized and collagen coated 8W10E+ ECIS culture chambers (see Methods and Figure 5 of Online Supplement) were treated with Wnt5A and IL-1β. Resistance of HCAEC monolayers was continuously recorded in Ohms every 5 min at multiple frequencies ranging from 62.6 Hz to 64 kHz, normalized to its value at time zero, and plotted with respect to time. Resistance measured from duplicate wells were grouped and averaged to plot as a single curve with error bars representing the SD. Data shown are the resistance measurements conducted at 4000 Hz from 4 independent experiments. (A) Un transfected HCAEC. Black, Untreated; Green, Wnt5A; Purple, IL-1β. (B) Ryk siRNA transfected HCAEC. Black, Untreated; Green, Wnt5A; Purple, IL-1β.
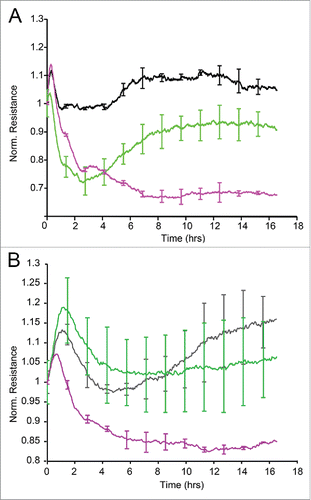
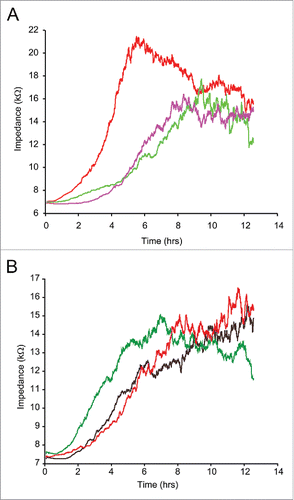
Figure 6. ECIS assisted wound healing assay. Uniform confluent monolayers of HCAEC cultured in stabilized and collagen coated single electrode 8W1E ECIS culture chambers (Methods) were treated with Wnt5A and IL-1β either alone or in combination with the ROCK inhibitor Y-27632 for 3 h. At time 0 h, a defined wound with a diameter of 250 µm was created in each of the 8 wells by applying an elevated electric field at 1400 μA, 60 kHz for 20 sec. Immediately after wounding, impedance of HCAEC was continuously recorded in Ohms every 5 min at multiple frequencies ranging from 62.6 Hz to 64 kHz and plotted with respect to time. Data shown represent the measurements conducted at 4000 Hz from 4 independent experiments. (A) HCAEC without ROCK inhibition. Red, Untreated; Purple, Wnt5A; Green, IL-1β. (B) HCAEC with ROCK inhibition. Black, Control with Y-27632; Red, Wnt5A+Y-27632; Green IL-1β+Y-27632.