Figures & data
Figure 1. BM-MSCs promote PC-3 Migration, Invasion and Adhesion. PC-3 (A) migration toward BM-MSCs in co-culture (****p < 0.0001), (B) migration toward BM-MSC CM (****p < 0.0001), (C) invasion toward BM-MSC CM (****p < 0.001), (D) adhesion to BM-MSC CM (*****p < 0.0001), (E) cell count after 72 h culture in BM-MSC CM (n.s.). Number of migrated and invaded cells reported are mean ± SD of 5 representative fields on the membrane. Proportion adherence reported is mean ± SD (n = 3) of the ratio of adherent to input cells as determined by cell viability (MTS) assay. Cell count reported is mean ± SD (n = 3) of cell viability (MTS). SFM: serum free media, MSC CM: BM-MSC conditioned media.
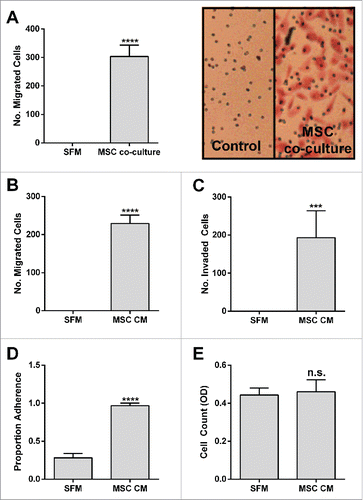
Figure 2. Initial proteomic characterization of BM-MSC secretome (A) BM-MSC-derived conditioned media (CM) was subjected to ultracentrifugation (50,000 × g for 3 hrs) and separately to variable molecular-weight exclusion filters (Centricon) by centrifugation. The starting CM, UC-derived supernatant (UC), and Centricon filtered (F) and retained (R) fractions were tested in a PC3 migration assay. (B) Concentrated CM (400 mL to 200 μl through a 100 kDa Centricon filter; solid line) was fractionated by size exclusion chromatography (gray line: MW standards). (C) Fractions corresponding to protein peaks in (B) were assessed for PC-3 chemotactic activity. The chemotactic activity in Fx1 and Fx2 was significantly increased relative to SFM (***p < 0.001). (D) Polypeptides from Fx1 were resolved on a 4–12% gel (Invitrogen), and silver stained (SilverQuest, Invitrogen) according to manufacturer's instructions. The bands were excised from the gel and subjected to mass spectroscopic (LC/MS/MS) analysis at Tufts Core Facility. Thrombospondin-1 (TSP-1) was identified from the 140 kDa band (arrow). (E) CM was incubated with 10 μg of α-TSP-1 antibody (IgG1κ, Invitrogen Life Technologies) or isotype control (Iso) antibody, immune complexes cleared by adding protein G-coupled agarose. TSP-1 (15 µg/ml), courtesy Jack Lawler, Beth Israel Deaconess Medical Center, was used for putback assay into TSP-1 depleted CM. Supernatants were tested in the PC-3 migration bioassay. TSP-1 immunodepletion reduced bioactivity, but TSP-1 putback did not recover activity. Number of migrated cells reported are mean ± SD of 5 representative fields on the membrane. Fil: Filtrate obtained by initial concentration of CM. SFM: serum-free medium control.
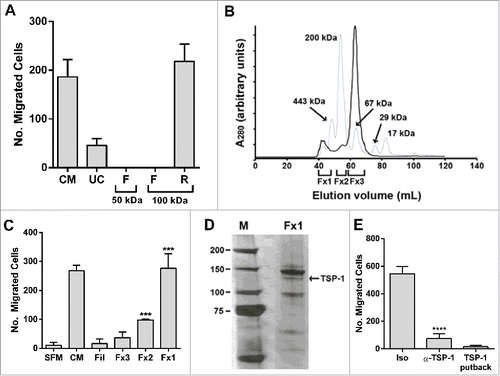
Figure 3. Fibronectin fragments are identified as the putative chemotaxin. (A) 500 mL of BM-MSC CM was subjected to heparin affinity chromatography (as described in methods). PC-3 cell migration assay was performed on load, eluate and flow through demonstrating that bioactivity was recovered in the eluate (****p < 0.0001 compared to SFM). (B) Concentrated heparin eluate (black line) was fractionated on size exclusion chromatography (gray line: molecular weight standards) and (C) resulting fractions were tested in a PC3 migration assay. Peak activity was found in Fx21 (****p < 0.0001). (D) Polypeptides from Fx14-21 were resolved on a 4–12% gel (Invitrogen Life Technologies), and silver stained (SilverQuest, Invitrogen) according to manufacturer's instructions. The bands were excised from the gel and subjected to mass spectroscopic (LC/MS/MS) analysis (Taplin Mass Spectrometry Facility, Harvard). A fragment of fibronectin was identified from the 140 kDa band (arrow). Number of migrated cells reported are mean ± SD of 5 representative fields on the membrane. SFM: serum-free media, CM: BM-MSC conditioned media, FN: fibronectin.
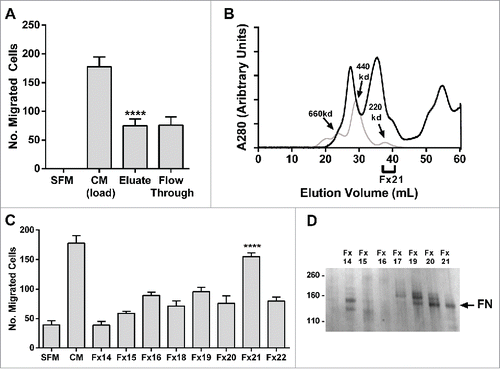
Figure 4. The classic fibronectin receptors α5 and β1 integrin mediate the migratory and adhesive behavior of prostate cancer cells to the BM-MSC secretome and fibronectin and its fragments. α5 or β1 integrin neutralization significantly impairs PC3 migration to (A) MSC CM (migration (left): ****p < 0.0001 and ****p < 0.0001; adhesion (right): ***p < 0.001 and ***p < 0.001 compared to isotype control) and (B) human plasma fibronectin (FN - MP Biomedicals) (migration (left): ****p < 0.0001 and ****p < 0.0001; adhesion (right): ****p < 0.0001 and ***p < 0.001 compared to isotype control), (C) Shrna knockdown of α5 significantly impairs migration (CM: ****p < 0.0001, FN: ****p < 0.0001) and adhesion (CM: ***p < 0.001, FN:***p < 0.001) to BM-MSC CM and FN. (D) Three commercial preparations of FN including FN i, which shows a restricted full length band on western, and FN ii and iii which contain fragments as well as proteolytically derived FN iv and v (30 minutes of trypsin and chymotrypsin digestion of FN i followed by quenching by PMSF) were tested in PC3 adhesion and migration assay. Although adhesion is consistently observed, only FN preparations with fragments induce migration. Integrin neutralizations were performed as described in methods. Number of migrated cells reported are mean ± SD of 5 representative fields on the membrane. Adhesion is reported as proportion adherence, mean ± SD (n = 3) of the ratio of adherent to input cells as determined by cell viability (MTS) assay, or directly as OD or RFU as determined by MTS or AlamarBlue (ThermoFisher). SFM: serum free media, MSC CM: BM-MSC conditioned media, Iso: isotype control (anti-α4), FN: fibronectin.
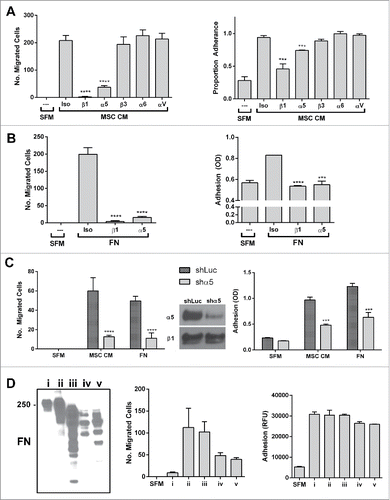
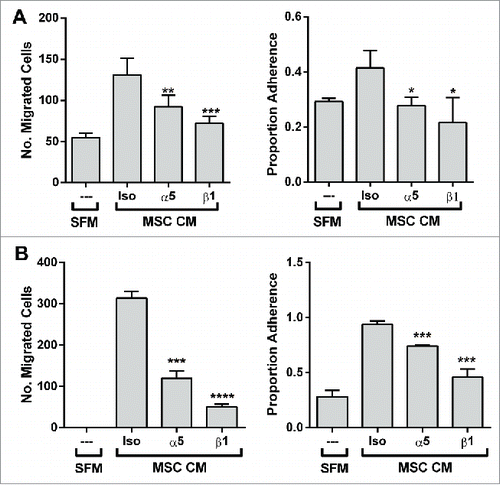
Figure 5. The migratory and adhesive response of other prostate cancer lines with α5-surface expression to the BM-MSC secretome is also mediated by α5 and β1 integrins. Neutralization of integrins α5 and β1 significantly impairs (A) LNCaP migration (α5: **p < 0.01, β1: ***p < 0.001) and adhesion (α5: *p < 0.05, β1: *p < 0.05) to BM-MSC CM and (B) DU-145 migration (α5: ***p < 0.001, β1: ****p < 0.0001) and adhesion (α5: ***p < 0.001, β1: ***p < 0.001) to BM-MSC CM. Integrin neutralizations were performed as described in methods. Number of migrated cells reported are mean ± SD of 5 representative fields on the membrane. Adhesion is reported as proportion adherence, mean ± SD (n = '3) of the ratio of adherent to input cells as determined by cell viability (MTS) assay. SFM: serum free media, MSC CM: BM-MSC conditioned media, Iso: isotype control (anti-α4).