Figures & data
Figure 1. Validation of LRP-1-silencing efficiency and purification of human recombinant RAP. (A) Purified recombinant RAP was assessed by SDS-PAGE followed by CBS and immunoblotting using anti-RAP antibody (IB). (B) Total RNAs were purified from FTC-133 cells transfected with non-silencing shRNA (shCTRL) or shRNA targeting LRP-1 (shLRP-1). The transcriptional level of LRP-1 was assessed by reverse transcription followed by a real-time PCR. β-actin primers were used as a normalization control. Graph represents the relative LRP-1 expression in percent. *, P< 0.05 (C) Whole-cell extracts from each clonal cell were subjected to immunoblot analysis under non-reducing conditions with anti-LRP-1 α-chain (8G1) and β-chain (5A6) antibodies. β-actin antibody was used for normalization.
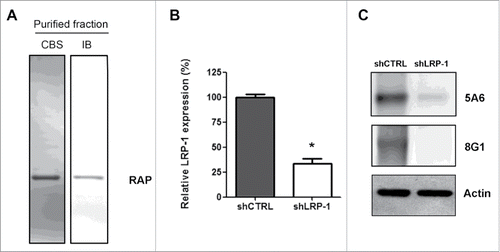
Figure 2. Thyroid carcinoma cell migration on 3D collagen matrix is drastically altered by LRP-1 blockade or silencing. FTC-133 cells were cultured for 24 h within 3D collagen I matrix, and then were tracked by time-lapse videomicroscopy. (A) Cell migration speed (µm/h) was assessed during the first 6 h and the last 6 h of culture and data represent the average of 3 independent experiments (at least 25 cells were tracked per experiment). Error bars represent SEM. ***, P < 0.001 significant difference. (B) Distance of migration covered by cells during the first 6 h and the last 6 h. Data represent the average of 3 independent experiments (at least 25 cells tracked per experiment). (C) Quantitative analysis of 3D FTC-133 cell trajectories during the last 6 h of culture. Each color corresponds to a distinct cell.
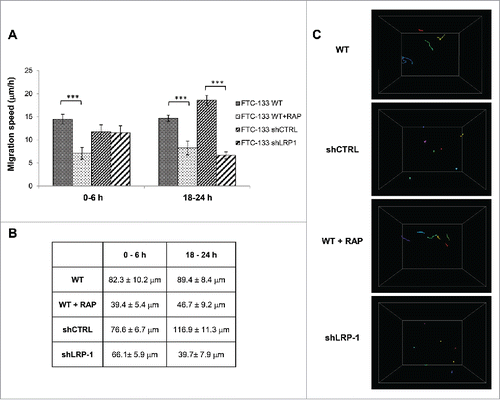
Figure 3. LRP-1 blockade or silencing impacts the FTC-133 carcinoma cell morphology on 3D collagen matrix. (A) Cells were seeded within 3D type I collagen matrix. Typical morphology of cells cultured within 3D matrix was obtained by phase contrast microscopy. Arrows show examples of nascent thin projections. Bar: 100 μm. (B) Percentage of round cell was calculated by using 30 isolated cells (180 min after seeding) for each condition in 3 independent experiments. ***, P < 0.001. (C) Ratio (length/width) was calculated using Image J software by using 20 isolated cells (60 min and 180 min after seeding) for each condition in 3 independent experiments. ***, P < 0.001.
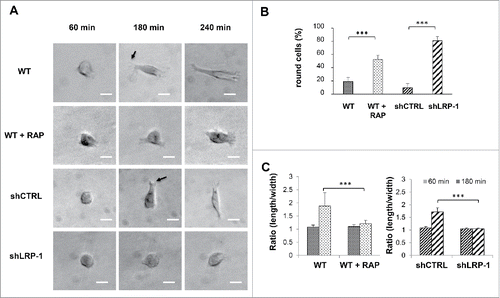
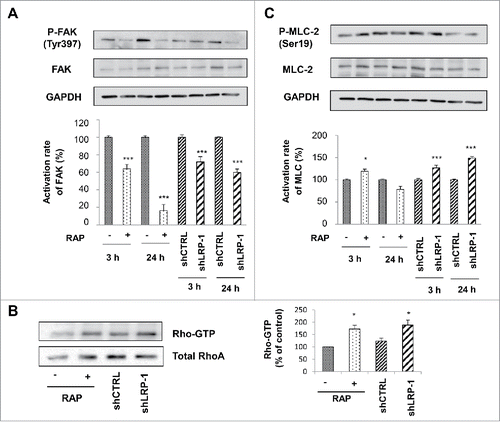
Figure 4. FAK and MLC-2 Phosphorylation and Rho activation are LRP-1 dependent in 3D matrix. Wild-type FTC-133 (treated with or without RAP) and clonal cells (shCTRL and shLRP-1) were seeded in 3D collagen matrix for 3 h and 24 h. Whole-cell extracts were subjected to Western-blot analysis to evaluate the level of FAK activation (A) and the phosphorylation of MLC-2 (B). The respective phosphorylation rates of FAK (A) and MLC-2 (B) were determined as intensity ratios of phospho-protein to corresponding pan-protein and expressed in relative units +/− SD, with a value of 100% ascribed to wild-type cells (right panels). GAPDH antibody was used as a loading control. (C) Cell lysates were incubated with GST-RBD beads. The bound RhoA was detected with a monoclonal anti-RhoA antibody (Top). The relative amount of total RhoA in the cell lysates were assessed by using a monoclonal antibody against RhoA (Bottom). Quantitative analysis of the GTP-RhoA associated with RBD beads was obtained by densitometry. The amount of RhoA bound to RBD was normalized to the RhoA content of cell extracts. *, P<0.05; ***, P < 0.001.