Figures & data
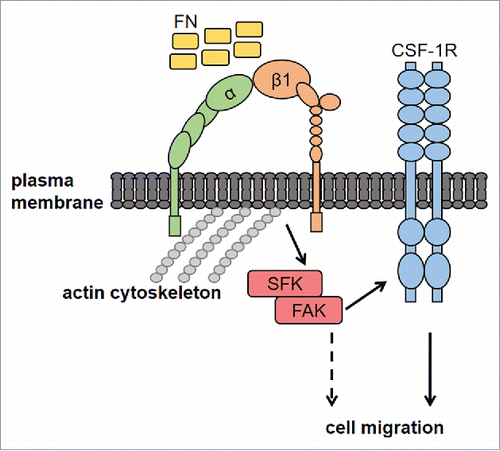
Figure 1. Effects of FN on migration and FAK in macrophages. Dose-response effect of FN on the migration of murine BAC1.2F5 macrophages. (A) Cells were cultured in the absence of CSF-1 for 18 hours and then subjected to migration assay toward DMEM containing or not FN at the indicated concentrations or PL (10 μg/ml). Migrated cells were counted. Histograms represent means ± SEM of data from 4 independent experiments each performed in triplicate. Student's t test: *, p< 0.05 versus untreated. Kinetics of FAK activation induced by FN in BAC1.2F5 cells. (B) Cells were cultured in the absence of CSF-1 for 18 hours, kept in suspension for 45 minutes and then plated on FN- or PL-coated dishes (10 μg/ml) for the indicated times. Protein lysates were then subjected to immunoprecipitation (IP) and/or immunoblotting (WB) with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. FN-induced macrophage adhesion. (C) Cells were cultured in the absence of CSF-1 for 18 hours and then let adhere on FN- or PL-coated coverglass (10 μg/ml) for the indicated times. Cells were then stained with anti-vinculin antibodies and analyzed by confocal microscopy. Cell area was then measured. Histograms represent means ± SEM of data from 2 independent experiments. Student's t test: *, p < 0.05 vs. FN. Examples of selected areas are indicated. FN induces macrophage migration and FAK activation in J774 murine macrophages as well as human primary macrophages. (D, F) Migration assay was performed as described in (A). Histograms represent means ± SEM of data from 2 independent experiments each performed in triplicate. Student's t test: *, p < 0.05 versus untreated; **, p < 0.01 vs. untreated. (E, G) Total cell lysates were subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 (E) or 2 (G) independent experiments are shown. Molecular weight markers are reported on the left of gels.
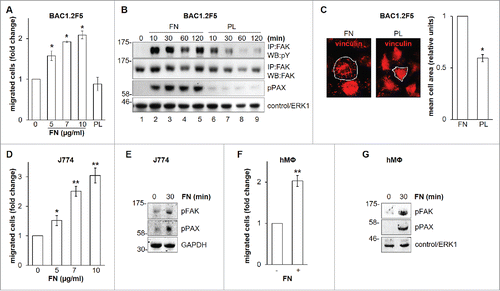
Figure 2. Effects of genetic or pharmacological inhibition of FAK on FN-induced macrophage migration. Genetic inhibition of FAK prevents FN-induced macrophage migration. (A-C) Cells were transfected with non-targeting control (NT) or FAK-specific (FAK) siRNA, incubated in complete medium for 48 hours and then cultured in the absence of CSF-1 (BAC1.2F5) or FBS (J774) for 18 or 24 hours, respectively. (A) Cells were then lysed and subjected to western blotting with the indicated antibodies. Numerical values represent the densitometric ratio FAK/NT (+/− SEM) relative to the FAK band (normalized for the densitometric value of the respective GAPDH band within the same gel) and are the average of data from 3 independent experiments. Likewise, representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. (B, C) Cells were subjected to migration assay toward DMEM containing or not 10 μg/ml FN. Histograms represent means ± SEM of data from 3 independent experiments performed in triplicate. Student's t test for paired samples; *, p ≤ 0.05; ns, not significant. Pharmacological inhibition of FAK prevents FN-induced macrophage migration. (D) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes and lysed (0) or plated on FN-coated dishes (10 μg/ml) for 30 minutes in the presence or the absence of PF-573228 (1 μM). Cells were then lysed and proteins subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. (E, F) Cells were cultured in the absence of CSF-1 for 18 hours and then treated or not with PF-573228 (1 μM) for 30 minutes before being subjected to migration assay toward DMEM containing or not FN at the indicated concentrations. Migrated cells were counted. Histograms represent means ± SEM of data from 3 (E) or 2 (F) independent experiments performed in triplicate. Student's t test: *, p < 0.05; **, p < 0.01; ns, not significant.
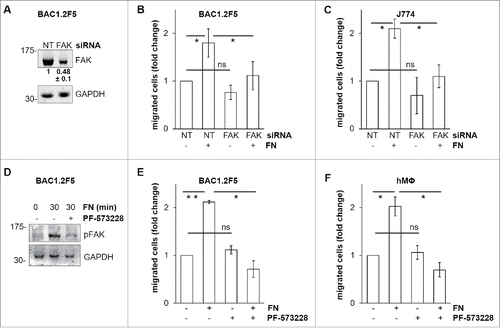
Figure 3. Mechanism of FN-induced FAK activation in BAC1.2F5 cells. Actin polymerization is necessary for FN-induced FAK phosphorylation. (A) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension in the presence or not of cytochalasin D (CD, 10 μM) before being plated on FN- or PL-coated (10 μg/ml) dishes for the indicated times. Protein lysates were subjected to immunoprecipitation (IP) and immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. SFK mediate FN-induced FAK phosphorylation. (B) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes in the presence or the absence of the SFK inhibitor PP2 (10 μM) before being plated on FN- or PL-coated (10 μg/ml) dishes for the indicated times. Protein lysates were subjected to immunoprecipitation (IP) and/or immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. SFK mediate FN-induced macrophage migration. (C) Cells were cultured in the absence of CSF-1 for 18 hours and then treated or not with the SFK inhibitors PP2 or SU6656 (10 μM) for 30 minutes before being subjected to migration assay toward DMEM containing or not FN (10 μg/ml). Migrated cells were counted. Histograms represent means ± SEM of data from 2 independent experiments performed in triplicate. Student's t test: *, p < 0.05; ns, not significant.
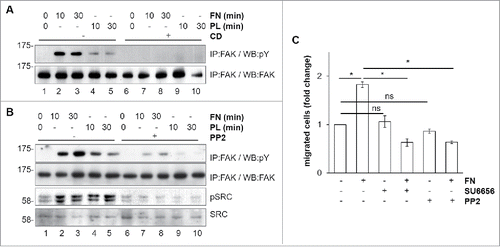
Figure 4. Involvement of CSF-1R in FN-induced macrophage migration. FN induces CSF-1R phosphorylation. (A-C) Cells were cultured in the absence of CSF-1 for 18 hours (A) or in the absence of FBS for 24 hours (B, C) and kept in suspension for 45 minutes and then lysed or plated on FN-coated (10 μg/ml) dishes for 30 minutes before lysis. Protein lysates were subjected to immunoblotting with the indicated antibodies. Molecular weight markers are reported on the left of gels. Representative images of WB from one out of 3 independent experiments are shown. Genetic inhibition of CSF-1R prevents FN-induced macrophage migration. (D-F) Cells were transfected with non-targeting control (NT) or CSF-1R-specific (CSF1R) siRNA for 48 hours and then cultured in the absence of CSF-1 for 18 hours (D, F) or in the absence of FBS for 24 hours (E) and subjected to migration assay toward FN (D, E) or lysed and subjected to immunoblotting with the indicated antibodies (F). (D, E) Histograms represent means ± SEM of data from 3 independent experiments performed in triplicate. Student's t test: **, p< 0.01. (F) Representative images of WB from one out of 4 independent experiments are shown. Molecular weight markers are reported on the left of gels. Pharmacological inhibition of CSF-1R prevents FN-induced macrophage migration. (G) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes and lysed or plated on FN-coated dishes (10 μg/ml) for 30 minutes in the presence or the absence of GW2580 (1μM). Cells were then lysed and proteins were subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. (H, I) Cells were cultured in the absence of CSF-1 for 18 hours and then were treated or not with GW2580 (1μM) for 30 minutes before being subjected to migration assay toward DMEM containing or not 10 μg/ml FN. Migrated cells were then counted. Histograms represent means ± SEM of data from 3 (H) or 2 (I) independent experiments performed in triplicates. Student's t test: *, p < 0.05; **, p < 0.01; ns, not significant.
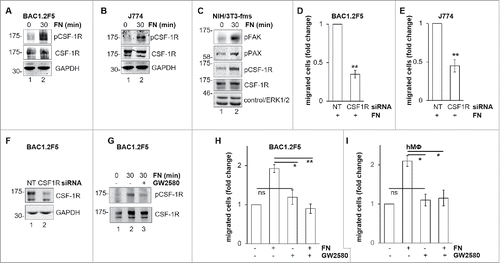
Figure 5. Involvement of FAK in CSF-1R activation induced by FN in BAC1.2F5 cells. Genetic inhibition of FAK decreases FN-induced CSF-1R phosphorylation. (A) Cells were transfected with non-targeting control (NT) or FAK-specific (FAK) siRNA for 48 hours, kept in suspension for 45 minutes and then lysed or plated on FN-coated (10 μg/ml) dishes for 30 minutes. Protein lysates were subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. Pharmacological inhibition of FAK prevents FN-induced CSF-1R phosphorylation. (B) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes and lysed (0) or plated on FN-coated dishes (10 μg/ml) for 30 minutes in the presence or the absence of PF-573228 (1 μM) before lysis. Proteins were subjected to immunoblotting with the indicated antibodies. Representative images of WB from one out of 3 independent experiments are shown. Molecular weight markers are reported on the left of gels. CSF-1R interacts with integrin β1 following adhesion on FN. (C) Cells were cultured in the absence of CSF-1 for 18 hours and then kept in suspension for 45 minutes and lysed (0) or plated on (10 μg/ml) FN-coated dishes for the indicated times (minutes) before lysis. Protein lysates were then subjected to co-immunoprecipitation (IP) and immunoblotting with the indicated antibodies. Representative images of WB from one out of 2 independent experiments are shown. CSF-1R co-localizes with B1 following adhesion onto FN. (D) Cells were cultured in the absence of CSF-1 for 18 hours, kept in suspension for 45 minutes and then plated on FN-coated coverglass (10 μg/ml) for 30 minutes. Cells were then stained with the indicated antibodies and analyzed by confocal microscopy. Representative images are from one out of 3 independent experiments. Scale bar: 10 μm.
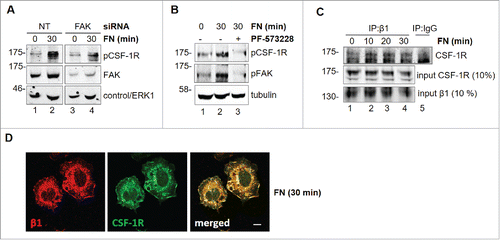
Figure 6. Proposed mechanisms of FN-induced macrophage migration through the SFK-FAK/CSF-1R pathway. Macrophage adhesion onto FN, through β1 integrin, activates the SFK/FAK complex, thereby leading to CSF-1R phosphorylation and migration. CSF-1R-independent migration induced by FN (dashed line) cannot be excluded, although not observed in our experiments. FN, fibronectin; CSF-1R, Colony-Stimulating Factor-1 Receptor; SFK, Src Family Kinases; FAK, Focal Adhesion Kinase; α and β1, integrin subunits.