Figures & data
Figure 1. Schematic representation of SgK269 and SgK223 structure. Amino acid substitutions in the pseudokinase domain relative to consensus sequence motifs in bona fide kinases (glycine-rich loop and DFG motif) are highlighted. Functionally-annotated tyrosine phosphorylation sites are also shown. SgK269 Y665 is known to regulate focal adhesion turnover. CH: predicted C-terminal α-helical region. For full details please refer to text.
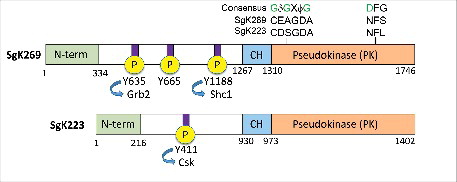
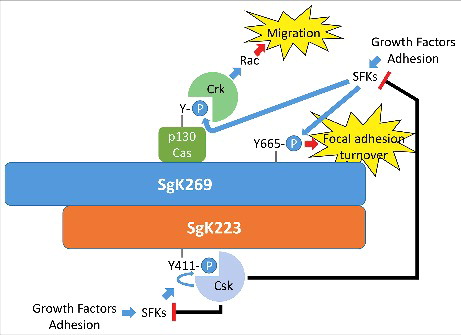
Figure 2. A potential role for SgK269/SgK223 heterotypic association in regulation of focal adhesion dynamics. Both SgK269 and SgK223 localize to focal adhesions, and SgK269 is known to associate with specific focal adhesion components. SgK269 also regulates focal adhesion turnover in a manner dependent on dynamic phosphorylation of Y665. Heterotypic association of SgK269 with SgK223, which recruits Csk, provides a potential mechanism for dynamic regulation of Src family kinases and hence focal adhesion turnover.