Figures & data
Figure 1. Actin-based protrusions in mesenchymal cells. (A) Schematic of the actin-based protrusions in a mesenchymal cell showing in red filamentous (F) actin. Lamellipodia, filopodia, (peripheral and dorsal) ruffles and circular dorsal ruffles (CDRs) are depicted. Boxes highlight that the geometry of F-actin distinguishes filopodia from all other actin-based cells protrusions formed on a 2D substrate. (B) Process diagram showing that 1) activation of the Arp2/3 complex requires two co-factors, namely a nucleation promoting factor (NPF) and pre-existing actin filaments (mother filaments), and 2) the Arp2/3 complex is an auto-catalytic actin nucleator because the product of the reaction (branched actin filaments) can be used as a substrate. (C) Dedicated pathways control actin nucleation by the Arp2/3 complex and formins thereby dictating the formation of different cell protrusions. Upstream regulatory Rho GTPases, NPFs and actin nulceators are shown. Question mark represents unknown formin-specific NPFs.
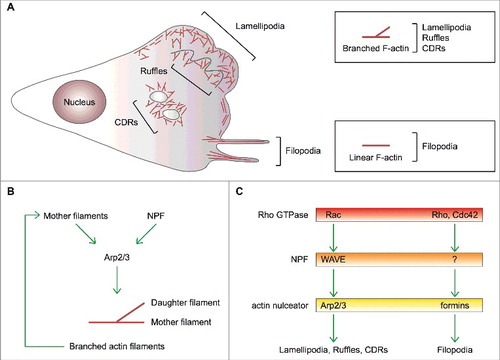
Figure 2. Mechanism of initiation of lamellipodia and ruffles. (A,B) Growth factors (GFs) activating receptor tyrosine kinases (RTKs) and other motogenic signals lead to the activation of the small GTPases Rac and Rho. (C) Active, GTP-bound Rho and Rac recruit mDia1 and the WAVE complex to the plasma membrane. Direct binding to Rho changes mDia1's conformation thereby converting it into the actin nucleation-proficient state. Similarly, a physical interaction between Rac and the WAVE complex results in the exposure of the VCA region of WAVE, which can then bind and prime the Arp2/3 complex. (D,E) mDia1 polymerizes linear actin filaments that can be used as a template by WAVE-primed Arp2/3 complex. (F) The Arp2/3 complex assembles the first actin branch thereby initiating lamellipodia and ruffles. Key proteins and complexes are not drawn in scale and are decoded in the box. Note that all WAVE-complex subunits but HSPC300, as well as the domains of WAVE, are depicted in the cartoon.
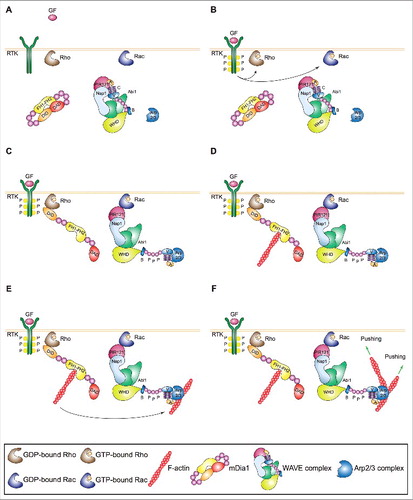
Figure 3. Mechanism of expansion of lamellipodia and ruffles. (A) Left: Process diagram showing how the Arp2/3 complex and WAVE and upstream regulators thereof sustain the expansion of lamellipodia and ruffles. Right: Cartoon depicting the enrichment of activated Rac and WAVE complex at the tip of lamellipodia and ruffles. The roles of WAVE as a 1) distributive polymerase promoting branched filament elongation, 2) tethering factor linking the actin network to the encasing plasma membrane and 3) nucleation promoting factor for the Arp2/3 complex are indicated. (B) The presence of active GTP-bound Rac within lamellipodia and ruffles is important for the enrichment and activity of the WAVE complex at the tip of these protrusions. The site-restricted cortical localization of the WAVE complex and the new branched actin filaments ensures persistent actin polymerization by the Arp2/3 complex in a narrow region close to the plasma membrane. This process and the proteins that regulate the elongation of the filaments' barbed ends pushing towards the plasma membrane control the protrusion of lamellipodia and ruffles, the latter indicated by green arrows. Key proteins and complexes are not depicted in scale and are decoded in the box. Note that all WAVE-complex subunits but HSPC300, as well as the domains of WAVE, are depicted in the cartoon. (C) Regulation of branched actin filament elongation within lamellipodia and ruffles. Inventory of the proteins that regulate either negatively (red box) or positively (green box) the filament elongation rate of the branched F-actin network. Question mark highlights that FMNL2 and FMNL3 might promote filament elongation only in some specific cell types.
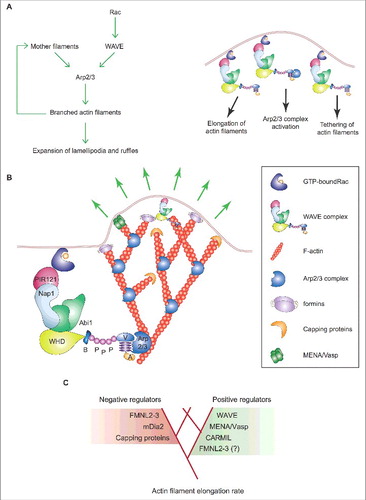
Figure 4. Lamellipodia and filopodia fulfill different exploratory functions but are not essential for mesenchymal cell migration. (A) Process diagram depicts the key players regulating actin polymerization for the formation of lamellipodia (left) and filopodia (right) and the main functions of either actin-based protrusion in mesenchymal cells migrating on a 2D uniform surface. Green and red lines denote activation and inhibition, respectively. The involvement of Rho proteins in filopodium formation has been recently reviewed [Citation122]. (B) Top: Stereotypical representation of actin-based lamellipodial and filopodial protrusions at the leading edge of mesenchymal cells (F-actin arrays and nuclei are highlighted in red and brown, respectively). Bottom: The main molecular features and the functions of lamellipodia and filopodia in mesenchymal cell migration are compared side to side. Ruffles are not indicated because they affect cell migration on 2D surfaces only indirectly.
![Figure 4. Lamellipodia and filopodia fulfill different exploratory functions but are not essential for mesenchymal cell migration. (A) Process diagram depicts the key players regulating actin polymerization for the formation of lamellipodia (left) and filopodia (right) and the main functions of either actin-based protrusion in mesenchymal cells migrating on a 2D uniform surface. Green and red lines denote activation and inhibition, respectively. The involvement of Rho proteins in filopodium formation has been recently reviewed [Citation122]. (B) Top: Stereotypical representation of actin-based lamellipodial and filopodial protrusions at the leading edge of mesenchymal cells (F-actin arrays and nuclei are highlighted in red and brown, respectively). Bottom: The main molecular features and the functions of lamellipodia and filopodia in mesenchymal cell migration are compared side to side. Ruffles are not indicated because they affect cell migration on 2D surfaces only indirectly.](/cms/asset/30c2c499-c906-4c86-a12a-94a082f4d6e6/kcam_a_1448352_f0004_oc.jpg)
