Figures & data
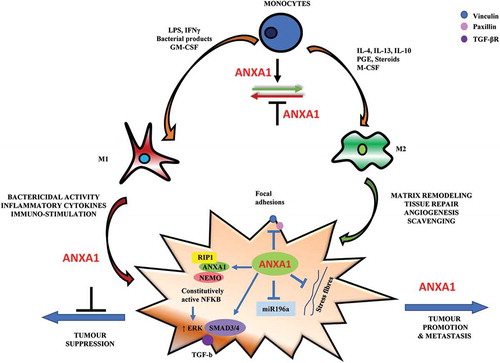
Figure 1. The potential influence of ANXA1 on macrophage polarization and breast cancer migration. Monocytes differentiate into polarized macrophage phenotype when exposed to different stimuli. In the presence of granulocyte-macrophage colony stimulating factor (GM-CSF), interferon (IFN-γ), lipopolysaccharide (LPS) and other bacterial products monocytes differentiate into M1 macrophages phenotype. In the presence of macrophage colony stimulating factor (M-CSF), interleukin (IL-4, IL10) and immune-suppressive agents (steroids, prostaglandins), monocytes differentiate to M2-phenotype. ANXA1 may be required for M2 macrophage polarization-dependent promotion of tumour growth and invasion. On the cancer cell, ANXA1 can promote breast cancer migration through its association with NEMO and RIP1 to constitutively activate NF-kB, which in turn can enhance ERK activation to promote migration. It can also enhance TGF-β/SMAD-dependent signalling to induce an epithelial mesenchymal transition and promote migration capability. ANXA1 has also been shown to inhibit focal adhesions and stress fibre formation which is related to lower adhesion and faster migration. ANXA1 can regulate the expression of microRNAs such as miR196a, an anti-migratory microRNA, which forms a negative feedback loop to enhance breast cancer migration.