Figures & data
Figure 1. Cyclin G mutants affect InR/TOR signaling. (A) The upper panel shows the size reduction of cycGHR7 mutants (right) compared to wild type (left). Underneath, a scheme of the InR/TOR signaling cascade is shown. (B-D) Small lipid droplets are seen in the fat body of a well-fed wild type larva (B). Droplet size increases in the case of amino acid deprivation (C). Similar sized droplets are seen in a cycGHR7 mutant larva under fed conditions (D). (B'-D') Double staining of lysosomes (green) and nuclei (magenta) in larval fat body is shown. In well-fed wild type larvae no signs of autophagy are seen (B'), whereas starved larvae reveal a conspicuous enrichment of a punctate lysosomal staining (C'), which is likewise detected in well-fed cycGHR7 mutant larvae (D').
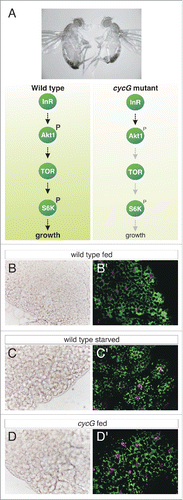
Figure 2. CycG interacts with the Wdb-PP2A complex and with Wrd. (A) Illustration of the heterotrimeric PP2A complex in Drosophila: The catalytic subunit Microtubule stars/Mts (PP2A-C) binds to the scaffold protein PP2A-29B (PP2A-A, called PR65 in mammals). Three regulatory subunits are known, PP2A-B called Twins/Tws (mammalian B55 aka PR55), and 2 PP2A-B′ subunits called Widerborst/Wdb (homolog of mammalian B56 aka PR61) and Well rounded/Wrd (homolog of mammalian PR72). (B) CycG binds the Wdb-PP2A complex. CycG proteins were immunoprecipitated (IP) from Drosophila Schneider (S2) cells using CycG antibodies, and the precipitates were probed with antibodies directed against the different subunits of PP2A (WB). The input lane contains 25% of the protein extracts used for the IPs and was used for a size comparison of the precipitated proteins. Size of proteins is given in kDa. (C–D) Yeast 2-hybrid assays were conducted for detection of direct protein interactions. Quantitative assays (measured in Miller Units, ordinate) were performed with full length CycG in pJG vector (1-566 in D) and the 5 subunits of PP2A in pEG vector (C). pEG-Wdb and pEG-Wrd were tested for binding to full length CycG (1-566, D), the N-terminal part (1-215, D) and the C-terminal part (215-566, D) of the CycG protein cloned into pJG vector. A scheme of CycG including the Cyclin domains is given, numbers correspond to amino acids. Empty vectors served as negative controls. Error bars denote standard deviation (n = 3 independent experiments). ***p < 0.001, **p < 0.01, ns: not significant according to Student's T-test.
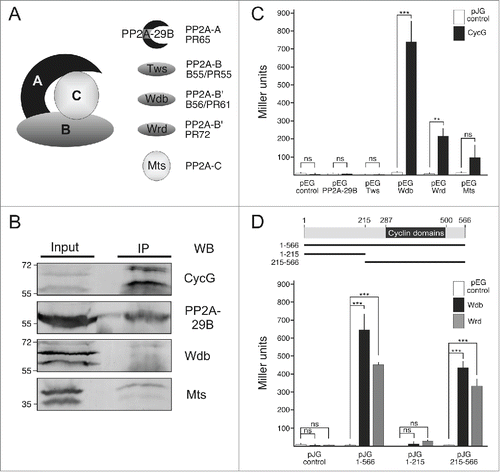
Figure 3. Analysis of wrd cycG double mutant phenotypes. (A) The weight reduction observed in cycGHR7 mutants compared to Oregon R wild type control was not significantly ameliorated in wrdko2 cycGHR7 doubly mutant males, in contrast to wdb cycG double mutants (wdb14 cycGHR7 / wdbdw cycGHR7). Citation16 Weight measurements of adult males are delineated in comparison to wild type flies which were set to 100%. The trans-heterozygous wdb14/wdbdw and the homozygous wrdko2 were included for comparison. Error bars denote standard deviation (n = 100 per genotype). ***p < 0.001, ns: not significant according to Student's T-test. (B) Statistical analyses of buoyancy tests repeated 5 times with 10 larvae each. Whereas Oregon R wild type, wdb14/wdbdw and wrdko2 larvae sink to the floor in 10% sucrose solution, cycGHR7 mutants float due to their increased fat content. This effect was rescued in an either wdb14 cycGHR7 / wdbdw cycGHR7 or wrdko2 cycGHR7 double mutant background, with only a small fraction of larvae still floating. Citation16 ***p < 0.001 according to Student's T-test. (C) Oil Red O staining reveals lipid droplet accumulation in larval oenocytes of cycGHR7 mutants compared to wild type control. Citation16 No accumulation of lipid droplet was observed in the oenocytes from the double mutant combinations wdb14 cycGHR7 / wdbdw cycGHR7 as well as wrdko2 cycGHR7 that match the control. Scale bar: 20µm.
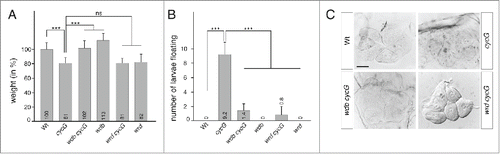
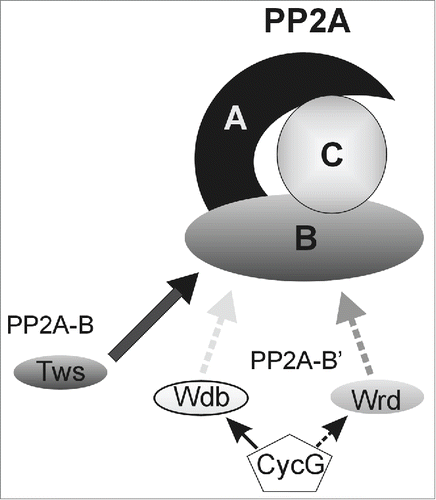
Figure 4. Model of CycG activity. By binding differentially to B′ subunits, CycG may influence their recruitment into PP2A trimeric complexes, thereby affecting PP2A specificity and activity in a context-dependent manner.