Figures & data
Figure 1. Auxin effects on Drosophila S2R+ cells. To evaluate potential toxicity of auxin, S2R+ cells were cultured in the presence of auxin at the indicated concentrations (mM). (A) No obvious effects of auxin on cell morphology were observed by imaging defined regions repeatedly at the indicated time points (hours) after auxin addition. Scale bar = 100 µm. (B) Cell counting revealed a slight inhibitory effect of 1 mM auxin on cell proliferation, while 0.3 mM did not appear to have a significant effect. Average cell numbers (+/− s.d., n = 3) at the indicated time points (hours) after addition of auxin (0, 0.3 and 1 mM) are shown.
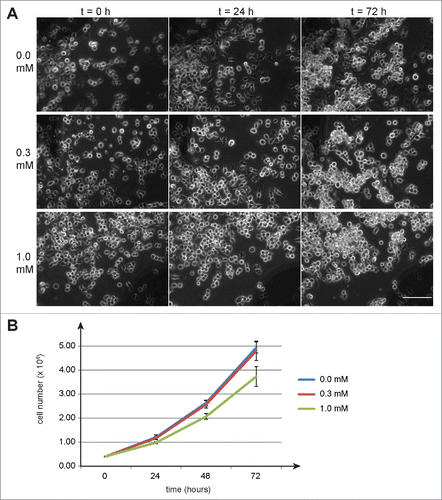
Figure 2. Auxin induced degradation in Drosophila S2R+ cells. (A) Scheme illustrating the characteristic features of the pMT-OsTIR1-P2A-H2B-aid-eyfp construct and of the auxin-inducible degradation system. The MtnA promoter (pMT) controls expression of an mRNA that includes the sequence of a “self-cleaving” 2A peptide (P2A). Therefore, the mRNA generates 2 distinct proteins. The first is the protein TIR1 from rice (OsTIR1) which includes an F box (F) that allows integration into an SCF ubiquitin ligase complex together with the endogenous Drosophila proteins Skp1, Cul1, Rbx1 and an E2 protein. The second protein is a histone H2B fusion protein with a C-terminal extension that consists of the auxin-inducible degron (aid) followed by EYFP (eyfp). In the presence of auxin, aid-containing proteins are recruited to OsTIR1, resulting in their polyubiquitinylation and proteasomal degradation. (B) The construct illustrated in panel A was transfected into S2R+ cells. Construct expression was either induced (+ CuSO4) or not induced (- CuSO4) before fixation and imaging. The strong nuclear YFP signals, which were observed in transfected and induced cells before addition of auxin (t = 0h), were no longer present when cells were fixed 3 h after addition of auxin (t = 3h). Scale bar = 20 µm. (C) By time lapse imaging the nuclear YFP signals were confirmed to disappear from transfected and induced S2R+ cells within less than an hour after addition of auxin. The upper images (t = 0h) were acquired immediately before and the lower images (t = 1h) one hour after auxin addition.
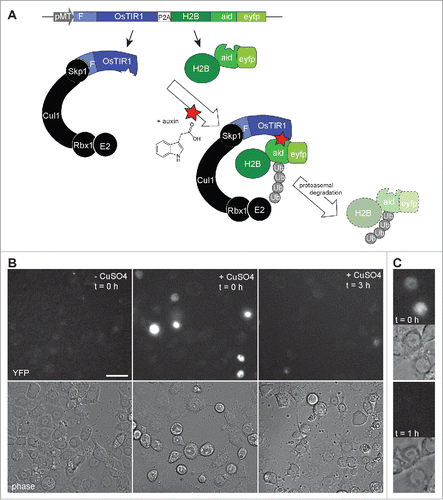
Figure 3. Auxin and OsTIR1 expression suppress Aid-Rux induced lethality during Drosophila development. (A) Schematic illustration of the experimental strategy used for the evaluation of the auxin-inducible degradation system during Drosophila development. Eggs with the indicated genotypes were collected on fly food with or without auxin. While complete developmental lethality was observed in the absence of auxin, the indicated number of adult flies eclosed in the presence of auxin. (B) The posterior wing compartments of en-GAL4>UASt-aid-rux, Ubi-OsTIR1 flies after development in the presence of auxin were observed to be severely reduced. (C) The posterior wing compartments of en-GAL4>UASt-aid-rux, UASt-OsTIR1 flies after development in the presence of auxin were observed to be abnormal as well. Abnormalities were more severe after development in the presence of 0.3 mM compared to 1 mM auxin. While the size of the posterior compartment was usually close to normal, it displayed a multiple wing hair phenotype, as clearly apparent in the high magnification view on the right. Scale bar = 0.4 mm.
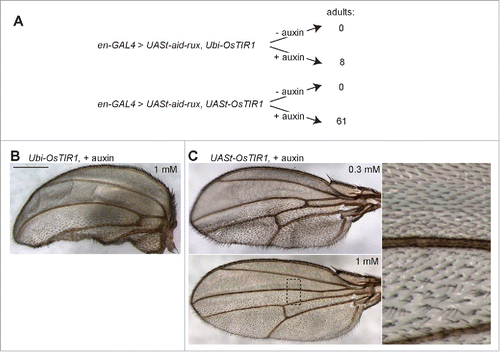
Figure 4. Aid-Rux depletion by auxin and OsTIR1 expression suppresses endoreduplication in wing discs. (A) Wing imaginal discs from third instar wandering stage larvae with en-GAL4>UASt-aid-rux and either UASt-OsTIR1 or Ubi-OsTIR1, as indicated, were fixed after development in the absence (−) or presence (+) of auxin. DNA staining revealed endoreduplicated nuclei at lower density in the posterior wing disc compartment of larvae grown in the absence of auxin. Boxed regions are shown at higher magnification in the bottom panels. The effects of aid-rux expression in the posterior compartment resulted in variable distortions of the wing imaginal discs, as evident in the example shown in the leftmost panel where the posterior compartment runs oblique across the disc. Scale bar = 50 µm. (B) Larvae with en-GAL4, tubP-GAL80ts>UASt-aid-rux, UASt-OsTIR1 were grown initially at 18°C. GAL4-mediated expression of the UASt transgenes was induced for 24 h at 29°C. Larvae were then transferred into liquid food without (- auxin) or with (+ auxin) auxin for the indicated time (hours). Wing imaginal discs were dissected and stained with anti-Rux, anti-Cyclin A and a DNA stain. Wing pouch regions with anterior and posterior compartment on the left and right side, respectively, are shown. Aid-Rux is degraded more rapidly in the presence of auxin. Scale bar = 10 µm.
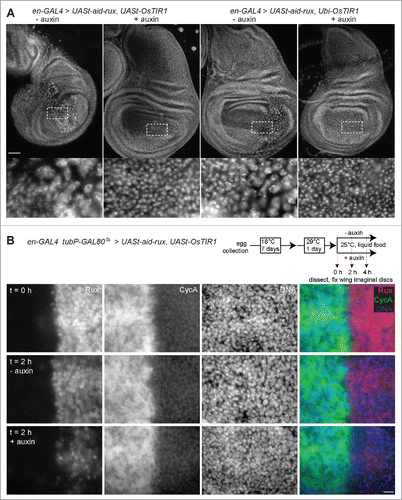
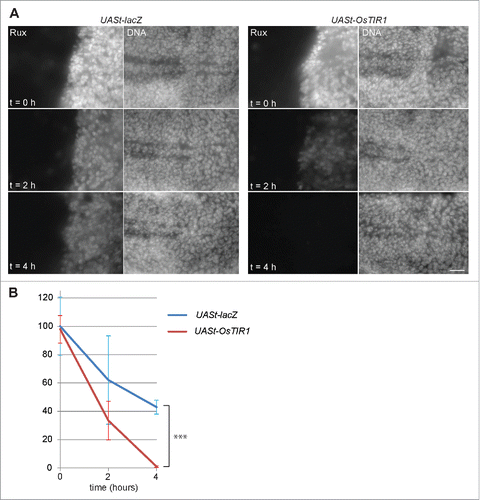
Figure 5. OsTIR1 does not cause auxin-independent Aid-Rux degradation. (A) en-GAL4, tubP-GAL80ts>UASt-aid-rux larvae with either UASt-lacZ or UASt-OsTIR1, as indicated, were grown initially at 18°C. GAL4-mediated expression of the UASt transgenes was induced for 24 h at 29°C. Larvae were then transferred for the indicated time (hours) into auxin containing liquid food. Wing imaginal discs were dissected and stained with anti-Rux and a DNA stain. OsTIR1 does not lower Aid-Rux levels before addition of auxin but results in rapid degradation after addition of auxin. Scale bar = 20 µm. (B) Anti-Rux signals in the posterior compartment of wing discs obtained in the experiment illustrated in (A) were quantified. Average signals were normalized with those observed in en-GAL4, tubP-GAL80ts>UASt-aid-rux, UASt-lacZ which were set to 100%; whiskers indicate s.d. (n = 3), *** p = 0.00013 (t-test).