Figures & data
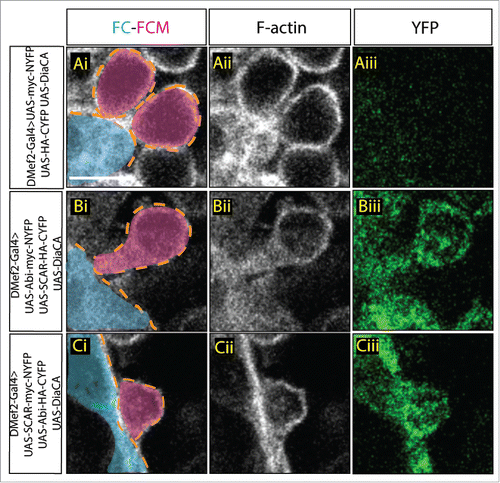
Figure 1. Visualization of Abi-SCAR complex formation using split YFP during myoblast fusion. Stage 15 embryo stained for F-actin (phalloidin, white) to label fusion site, and YFP (GFP antibody, green) to detect YFP reconstitution, FCM (magenta, false colored), and FC/myotube (turquoise, false colored). (Ai-Aiii) To visualize the background fluorescent level, UAS-myc-NYFP and UAS-HA-CYFP were expressed in the muscles under the control of muscles specific driver DMef2-Gal4. (Bi-Biii) UAS-Abi-myc-NYFP and UAS-SCAR-HA-CYFP; or (Ci-Ciii) UAS-SCAR-myc-NYFP and UAS-Abi-HA-CYFP were expressed in the muscles under the control of DMef2-Gal4. The reconstituted YFP signals indicate sites of Abi-SCAR interaction. Scale bar: 5 μm.
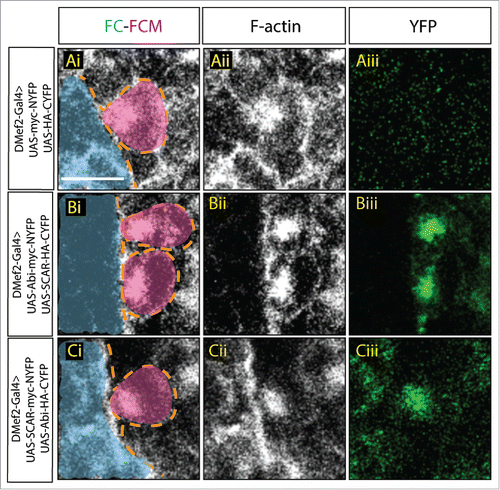
Figure 2. Increased SCAR activity results in fusion block. A. Three hemisegments from a stage 16 embryo. Embryos are stained for F-actin (phalloidin, white) to show the muscle pattern, and YFP (GFP antibody, green) to detect YFP reconstitution. (Ai–Aii) In control embryos, UAS-myc-NYFP and UAS-HA-CYFP were expressed in muscles under the control of DMef2-Gal4. Phalloidin staining shows wild-type muscle pattern. Background fluorescent level was visualized with antibody against GFP. UAS-Abi-myc-NYFP and UAS-SCAR-HA-CYFP (Aiii–Aiv) or UAS-SCAR-myc-NYFP and UAS-Abi-HA-CYFP (Av–Avi) were expressed in muscles under the control of DMef2-Gal4. Phalloidin staining shows impaired fusion and the actin focus at the fusion site. YFP shows the localization of Abi-SCAR interaction. (B) Muscle pattern from stage 16 embryos (antibody against Myosin Heavy Chain. white). Three hemisegments are shown from each embryo. One (Bi–Biv) or 2 copies (Bv–Bviii) of split-YFP labeled Abi or SCAR were expressed in the muscles under the control of DMef2-Gal4. Abi overexpression does not change muscle pattern. Increased expression of SCAR results in myoblast fusion block in a dosage dependent manner. Scale bar: 20 μm.
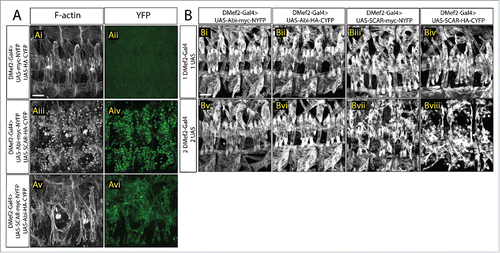
Figure 3. Constitutively active Dia changes Abi-SCAR localization and actin structure at the fusion site. Stage 16 embryo stained for F-actin (phalloidin, white) and YFP (GFP antibody, green), FCM (magenta, false colored), FC/myotube (turquoise/false colored). (Ai–Aiii) As control, constitutively active Dia (UAS-Dia.CA) was expressed together with UAS-myc-NYFP and UAS-HA-CYFP in the muscles under the control of DMef2-Gal4. Fusion is blocked in this context. Background YFP fluorescent level was visualized with antibody against GFP. (B-C) UAS-Dia.CA was expressed together with UAS-Abi-myc-NYFP and UAS-SCAR-HA-CYFP (Bi-Biii) or UAS-SCAR-myc-NYFP and UAS-Abi-HA-CYFP (Ci-Ciii) in the muscles. Actin morphology at the fusion site was visualized by Phalloidin staining of F-actin. Abi-SCAR complex was visualized by YFP reconstitution and was labeled by antibody against GFP. Scale bar: 5 μm.