Figures & data
Figure 1. Schematic view of our current understanding of the cellular mechanisms of apoptosis-induced compensatory proliferation (AiP). Undead cells can be induced by co-expression of the pro-apoptotic gene hid and the effector caspase inhibitor p35. Because P35 specifically inhibits the effector caspase DrICE (and Dcp-1; not shown), the initiator caspase Dronc cannot induce apoptosis (“undead” state), but remains active for apoptosis-independent functions. One of these functions is the activation of the transmembrane NADPH oxidase Duox, which generates extracellular ROS (eROS) such as superoxide and hydrogen peroxide. eROS activate and change the behavior of hemocytes to become proliferation-promoting.Citation3 This is accomplished through release of Eiger which activates the JNK pathway in undead and neighboring surviving cells. JNK stimulates the production of mitogens such as wingless (wg) (and dpp and spi; not shown) for AiP. In undead cells, JNK also induces expression of hid which sets an amplification loop in motion. Factors involved in apoptosis are indicated in blue, in JNK activation in green and in production of ROS in red. Question marks indicate unknown mechanisms.
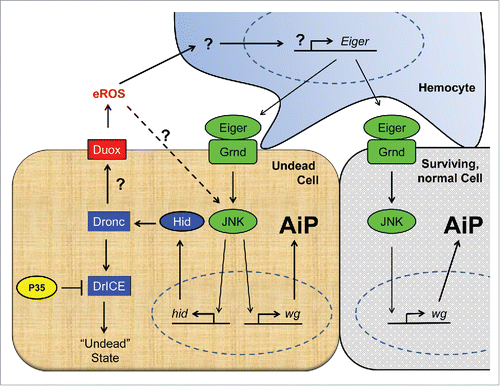
Figure 2. Undead tissue displays eROS-dependent hyperplastic overgrowth. Co-expression of hid and p35 in the developing eye imaginal disc using eyeless-Gal4 (ey-Gal4 UAS-hid UAS-p35 (ey>hid-p35)) promotes hyperplastic overgrowth of head cuticle with pattern duplications compared to wild-type control (A, B). Simultaneous expression of an extracellular catalase (hCatS), which neutralizes H2O2, suppresses overgrowth and normalizes the pattern of the adult head (C). Scale bars, 200μm.

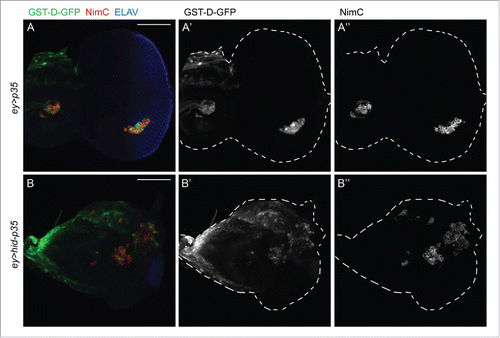
Figure 3. The redox reporter GST-D-GFP is expressed in hemocytes attached to both control and undead eye-antennal imaginal discs. Shown are eye-antennal imaginal discs of (A) ey-Gal4 UAS-p35 (ey > p35; control) and (B) ey-Gal4 UAS-hid UAS-p35 (ey > hid-p35; undead) genotype. Hemocytes are labeled using the NimC antibody (red in A, B; gray in A, “B”). GST-D-GFP is labeled in green in (A, B) and gray in (A’, B’). NimC and GFP labeling overlap. Shown also in (A, B) is labeling with ELAV which marks photoreceptor neurons in the posterior part of the eye disc. ey-Gal4 is only expressed in the anterior portion of the eye disc, but when expressing hid and p35, ey > Gal4 drives overgrowth of anterior tissue into the posterior part at the expense of photoreceptors. Scale bars, 100μm.