Figures & data
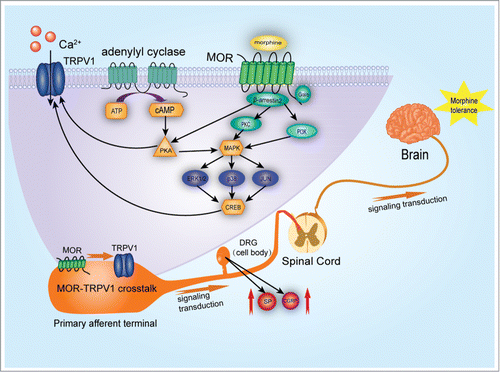
Figure 1. Signal transduction of TRPV1 activation in morphine induced antinociception, tolerance and dependence. By acting on μ- opioid receptors (MOR), primarily through Gai-subunits, morphine reduces adenylyl cyclase (AC) activity. AC catalyzes the conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), which regulates protein kinase A (PKA) or cyclic-nucleotide-gated ion channels. TRPV1 activation results in sensitization of TRPV1 responses through a β- arrestin2 and PKA-dependent manner. Decreased association of β-arrestin2 and constitutive phosphorylation of TRPV1 may underlie enhanced pain perception and hyperalgesia. Chronic administration of morphine activates the MAPK pathway, including ERK, p38 and JNK. This possibly occurs via protein kinase A (PKA), protein kinase C (PKC) and phosphatidylinositol 3-kinase (PI3K). The nuclear translocation of phosphorylated MAPK results in the phosphorylation of transcription factors, such as CREB and c-Jun. This leads to TRPV1 activation through modulation of neurotransmitters such as glutamate, CGRP and SP released from DRG neurons and further contributes to the antinociception, tolerance and dependence associated thermal hyperalgesia.