Figures & data
Table 1. Antibodies used in this study.
Figure 1. QGP-1 cells contain 5-HT and express Piezo2. Top row, epifluorescence images of Piezo2 and 5-HT immunohistochemistry with DAPI overlap. Middle row, omission of Piezo2 primary antibody eliminates Piezo2 labeling. Bottom row, omission of 5-HT antibody eliminates 5-HT labeling. Scale bar 50 µm.
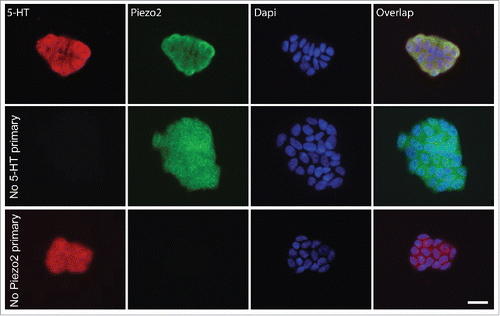
Figure 2. QGP-1 mechanosensitive currents are inhibited by the tarantula peptide D-GsMTx4 in a dose-dependent manner. QGP-1 cells were voltage-clamped and force was applied via graded membrane displacement by a piezo transducer driven glass probe. (A) Typical fast inward QGP-1 mechanosensitive currents in response to 3.5 µm membrane displacement in absence (control, black) and presence of 5 μM (red) and 10 μM (purple) D-GsMTx4. (B) Compared to the peak current in control, there was a significant decrease in peak current when Piezo2 was inhibited by 5 µM D-GsMTx4 (80.2 ± 18.4% inhibition, n = 4, *P < 0.05 by unpaired t-test with Welch's correction).
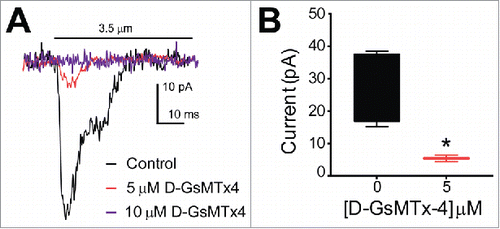
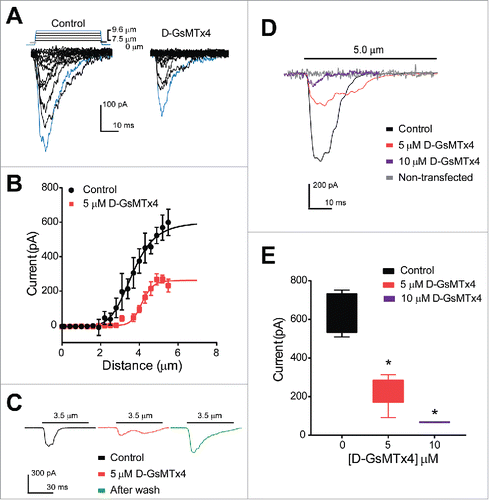
Figure 3. Human Piezo2 transiently transfected in HEK-293 cells produce mechanosensitive currents that are inhibited by D-GsMTx4. (A) A stepwise (0.3 µm step) increase in cell membrane deformation resulted in a set of fast activating and inactivating mechanically-induced inward currents, with blue traces highlighting current in response to a maximum stimulus, in both absence (Control) and presence (D-GsMTx4) of 5 µM D-GsMTx4. (B) Peak current-displacement relationship (n = 4) fitted by a two-state Boltzmann function in Control solution (Black, midpoint of 3.5 ± 0.1 µm) and perfused with 5 µM D-GsMTx4 (Red, midpoint of 4.2 ± 0.1 µm). (C) Inward currents elicited by 3.5 µm displacement in control solution (black) are inhibited by 55.7 ± 21.4% after application of 5 µM D-GsMTx4 (red) and washout (Green) restores peak current after exposure with 5 µM D-GsMTx4. (D) Typical inward currents evoked by a 5.0 µm displacement (Imax) in control solution (Black) and application of 5 µM D-GsMTx4 (Red) or 10 µM D-GsMTx4 (purple). A typical non-transfected HEK-293 cell response to mechanical stimulus is shown in gray. (E) Current-concentration whisker plot in presence of 0 (Black, −634.9 ± 53.0 pA), 5 µM (Red, −232.3 ± 37.2 pA) and 10 µM (Blue, −67.6 ± 0.9 pA) D-GsMTx4 (*P < 0.05 by unpaired t-test with Welch's correction).