Figures & data
Figure 1. SUMOylation inhibits the binding of 14–3–3τ to homomeric GluK2a receptors. (A, C) Western blot analyses of immunoprecipitates and cell lysates from HEK293T cells transfected with GluK2, Flag-tagged 14–3–3τ and other proteins as indicated. Whole-cell lysates were immunoprecipitated with an anti-Flag antibody and blotted with anti-Flag or anti-GluK2 antibodies. (B, D) Quantification of Western blots in (A) and (C), respectively. These values were determined by measuring the relative intensity of immunoprecipitated bands and their corresponding lysate (L) bands on Western blots and then normalized to and compared with first lane (control). The blot is representative of 4 independent experiments. Data are means ± SEM, **P < 0.01.
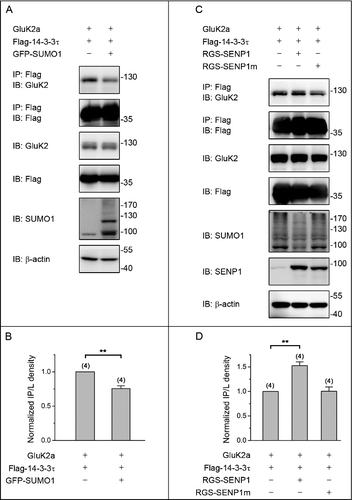
Figure 2. SUMO/deSUMOylation do not alter the level of mRNA and protein expression of GluK2a and 14–3–3τ. (A, C) Western blot analyses of cell lysates from HEK293T cells transfected with GluK2a, Flag-tagged 14–3–3τ and other proteins as indicated. The blot is representative of 3 independent experiments. (B, D) The relative 14–3–3τ and GluK2a mRNA levels in HEK239T cells which were transfected with GluK2a, Flag-tagged 14–3–3τ and other proteins as indicated by quantitative real-time PCR. Data are shown as means ± SEM from 3 independent experiments.
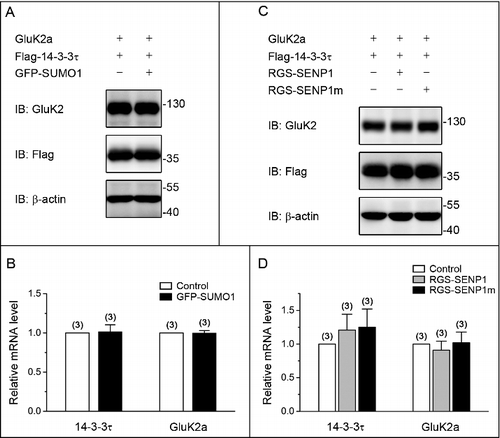
Figure 3. GluK2a SUMOylation does not change the binding of GluK2a to 14–3–3τ. (A) Western blot analyses of immunoprecipitates and cell lysates from HEK293T cells cotransfected with either GluK2 or SUMOylation mutant site of GluK2 (GluK2 k886R) with Flag-tagged 14–3–3τ. Cell lysates were prepared 24 h post-transfection and immunoprecipitated with anti-Flag antibody followed by Western blot with an anti-GluK2 or anti-Flag antibodies. (B) Quantification of Western blots in (A). The blot is representative of 3 independent experiments. Data are means ± SEM
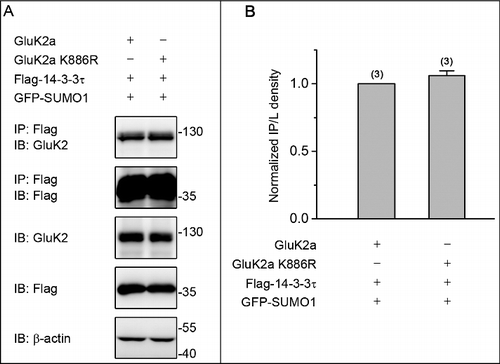
Figure 4. PKC SUMOylation repressed the binding of 14–3–3τ to GluK2. (A) Western blot analyses of immunoprecipitates and cell lysates from HEK293T cells cotransfected with Flag-tagged 14–3–3τ, GluK2, PKCα or the SUMOylation-deficient K465R PKCα, with or without GFP-SUMO1. Whole-cell lysates were immunoprecipitated with anti-Flag antibody and blotted with anti-Flag or anti-GluK2 antibodies. The blot is representative of 4 independent experiments. (B) Quantification of Western blots in (A). (C) HEK293T cells were transfected with plasmids as indicated. Whole-cell lysates were immunoprecipitated with an anti-GluK2 antibody and blotted with anti-Flag or anti-GluK2 antibodies. (D) Quantification of Western blots in (C). The blot is representative of 3 independent experiments. Data are means ± SEM, *P < 0.05, **P < 0.01.
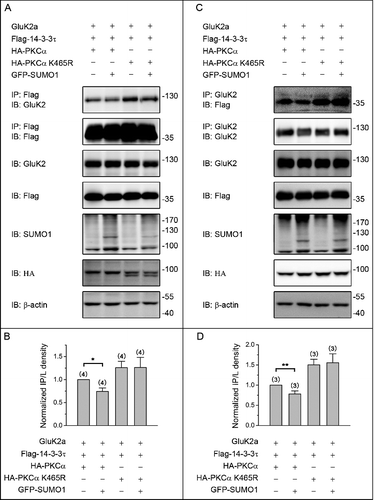
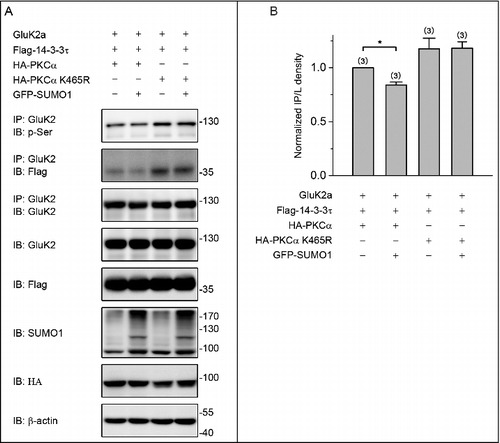
Figure 5. PKC SUMOylation decreases the level of GluK2a phosphorylation. (A) Western blot analyses of immunoprecipitates and cell lysates from HEK293T cells cotransfected with Flag-tagged 14–3–3τ, GluK2, PKCα or the SUMOylation-deficient K465R PKCα, with or without GFP-SUMO1. Whole-cell lysates were immunoprecipitated with an anti-GluK2 antibody and blotted with anti-phospho-(Ser), anti-Flag or anti-GluK2 antibodies. The blot is representative of 3 independent experiments. (B) Quantification of Western blots in (A). Data are means ± SEM, *P < 0.05.