Figures & data
Table 1. Voltage-dependent gating parameters of each group in a heterologous expression system
Figure 1. Expression defect for R1512W channels in Q1077del and Q1077 backgrounds and rescue for R1512W/Q1077del by H558R and mexiletine (MEX). (a) Whole-cell current traces from representative R1512W and WT channels in Q1077del and Q1077 backgrounds and R1512W/Q1077del with MEX or H558R. (b) Summary of Na+ current (INa) density in R1512W and WT channels in Q1077del and Q1077 backgrounds and R1512W/Q1077del with MEX or H558R. (c)Comparison of Na+ current (INa) density in R1512W/Q1077del, R1512W/Q1077del with MEX, R1512W/Q1077del/H558R and WT channels. The current amplitude was normalized to the membrane capacitance for each cell. *p value below 0.05/9 = 0.0055 (Bonferroni correction) indicates the INa density was significantly different compared R1512W without MEX to with MEX and WT in Q1077del background. **p < 0.0055 indicates the INa density was significantly different compared R1512W without MEX to with MEX and WT in Q1077 background. The INa density of R1512W was not significantly different compared to WT in Q1077del/H558R background
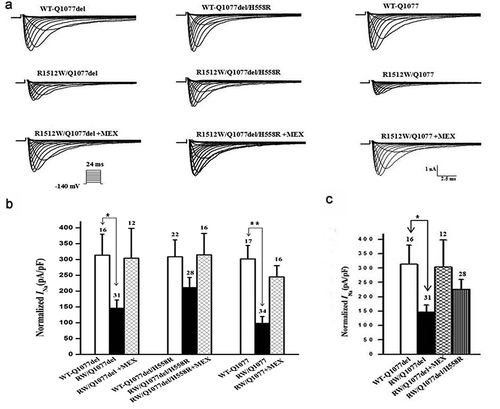
Figure 2. Voltage-dependent gating for R1512W and WT channels in Q1077del with and without MEX. (a) Voltagedependence of activation for R1512W and WT with and without MEX. The voltage clamp was 24 ms step depolarization to different potentials in increments of 10 mV from holding potential of – 140 mV (see insert). (b) Steady-state availability from inactivation for R1512W and WT with and without MEX. (c) Recovery from inactivation for R1512W and WT channels with time on a log scale to better show the early time course of recovery. (d) Intermediate inactivation for R1512W and WT channels with and without MEX. The activation and inactivation midpoints, intermediate inactivation and recovery from inactivation are not significantly difference between WT and mutant in Q1077del splice variant background. Insects: diagrams of voltage protocols. Values are means ± SE; n and fit parameters are given in
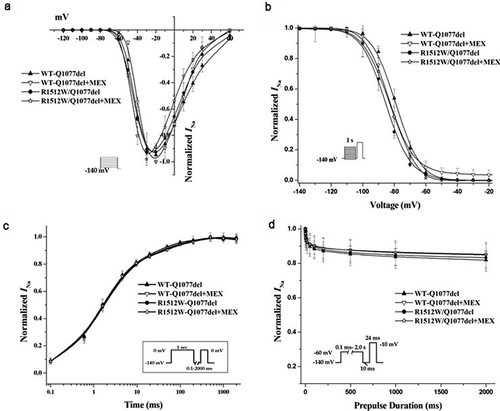
Figure 3. Voltage-dependence of activation (a), inactivation (b), recovery from inactivation (c), and intermediate inactivation (d) for R1512W and WT channels with and without MEX as in Figure 3 except in the Q1077 background. The activation and inactivation midpoint are not difference between WT and mutant channels. However, the slower time constants of recovery and enhanced intermediate inactivation were showed for R1512W compared with WT with and without MEX in the Q1077 splice variant background. # p < 0.05 indicates intermediate inactivation were significantly different compared R1512W and WT in Q1077 background
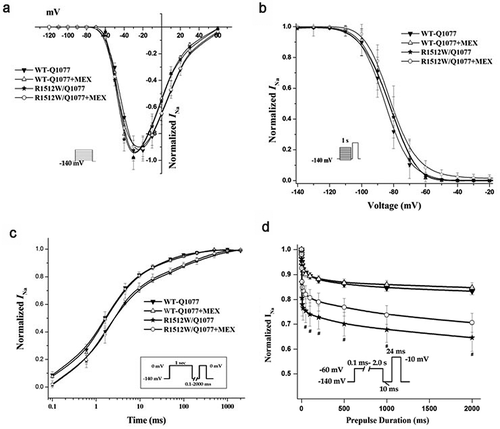
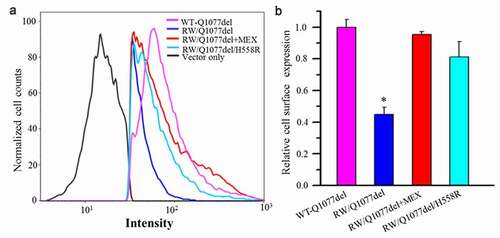
Figure 4. Flow cytometric analysis of cell surface expression of the HA-tagged SCN5A WT and mutant channels. HEK293 cells transiently transfected with HA-tagged WT-Q1077del, HA-tagged R1512W/Q1077del, HA-tagged R1512W/Q1077del+MEX, HA-tagged R1512W/Q1077del/H558R, respectively. After forty-eight hours of transfection with or without MEX treatment, the cells were harvested and stained by FITC-conjugated anti-HA antibody for quantitative the plasma membrane expression of HA-tagged channels by flow cytometry. (a) The Intensity of HA-tagged WT-Q1077del, HA-tagged R1512W/Q1077del, HA-tagged R1512W/Q1077del+MEX and HA-tagged R1512W/Q1077del/H558R. (b) Quantitation data to show the percentage of counted cells which the plasma membrane expressed HA-tagged WT-Q1077del, HA-tagged R1512W/Q1077del, HA-tagged R1512W/Q1077del+MEX, HA-tagged R1512W/Q1077del/H558R, respectively. *p < 0.001, compared with WT