Figures & data
Figure 1. DCtermD effect over CaV1.2Δ1820: (a) Representative whole-cell L-type Ba2+ current traces from AD293 cells overexpressing the truncated version of CaV1.2 channel (CaV1.2Δ1820) with or without the DCtermD. Currents elicited by a voltage step protocol from − 60 to +50 mV in 10-mV increments, Vh = −80 mV. (b) Summary peak current I/V plots (mean ± SEM) obtained from currents family as shown in (A); black lines represent the best fit to a Boltzmann equation. c) Graph shows the mean ± SEM of the peak Ba2+ current density. d) Graph shows the mean ± SEM of the midpoint of activation. In every panel, control cells are represented with filled circles and cells co-expressing the DCtermD with empty circles. *p < 0.05 with respect to control, n = 8 for control and n = 7 for DCtermD.
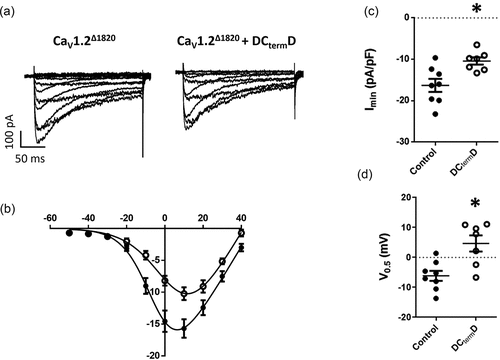
Figure 2. DCtermD modifies VDI and CDI of CaV1.2Δ1820: (a) Graph showing the residual of current after 200 ms during a 250-ms depolarization pulse (R200, mean ± SEM, n = 8 for control and n = 7 for DCtermD) versus command voltage of L-type Ba2+ currents AD293 cells overexpressing the CaV1.2Δ1820 channel with (empty circles) or without (filled circles) the DCtermD. Slower inactivation rates result in higher R200 values. (b) Representative trace of the IBa (black line) and ICa evoked by a 0 mV depolarizing pulse. Both currents were scaled to the same amplitude for comparison. (c) Graph shows the mean ± SEM of CDI fraction (n = 5 for control and n = 6 for DCtermD). *p < 0.05 with respect to control.
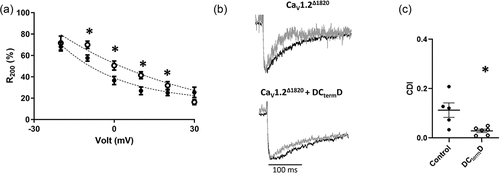
Figure 3. DCRD peptide displaces DCtermD from CaV1.2Δ1820 BRET titration curve from AD293 cells co-transfected with increasing amounts of CaV1.2Δ1820-YFP with (gray circles) or without (black circles) the DCRD peptide coding sequence, and a constant amount of DCtermD-RLuc as the energy donor. White circles denote experiments were the DCtermD without the RLuc was used. Saturation of the BRET curve indicates specific interaction between the DCtermD and the channel pore, whereas the low linear BRET signal denoted absence of interaction. Dashed lines represent the best fit to a hyperbolic curve or a linear fit respectively. Data from five experiments are included in the graph.
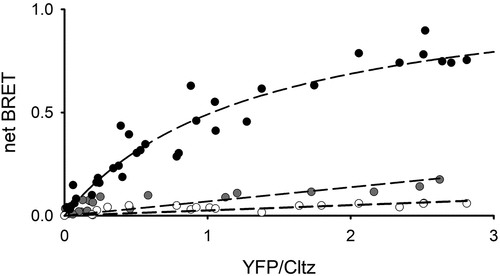
Figure 4. Effect of the DCRD peptide over CaV1.2Δ1820. (a) Representative whole-cell L-type Ba2+ current traces from AD293 cells overexpressing the CaV1.2Δ1820 channel with or without the DCRD peptide. Currents elicited by a voltage step protocol from − 60 to +50 mV in 10-mV increments, Vh = −80 mV. (b) Summary peak current I/V plots (mean ± SEM) obtained from currents family as shown in (A); black lines represent the best fit to a Boltzmann equation. (c) Graph shows the mean ± SEM of the peak Ba2+ current density. (d) Graph shows the mean ± SEM of the midpoint of activation. In every panel, control cells are represented with filled circles and cells co-expressing the DCRD peptide with empty circles. Control data set is the same used in . *p < 0.05 with respect to control, n = 8 for control and n = 7 for DCRD peptide.
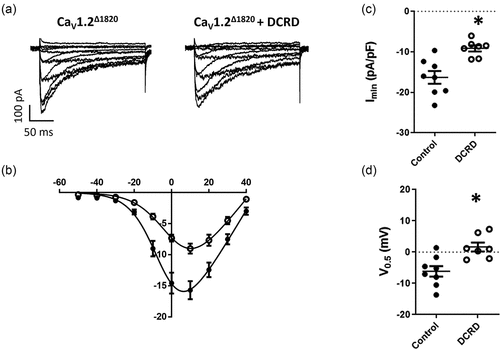
Figure 5. No effect of DCRD peptide over CaV1.2Δ1820 calcium-dependent inactivation: (a) and (c) Representative trace of CaV1.2Δ1820 barium current (black line) and calcium current evoked by a 0 mV depolarizing pulses from control AD293 cells (a) or AD293 cells overexpressing calmodulin (c). IBa and ICa currents were scaled to the same amplitude for comparison. (b) and (d) Graph shows the mean ± SEM of CDI fraction from AD293 control cells (n = 5) (b) or AD293 cells overexpressing calmodulin (n = 8 for control, n = 6 for DCtermD, and n = 6 for DCRD peptide). The control data set of panel B is the same used in . *p < 0.05 with respect to control.
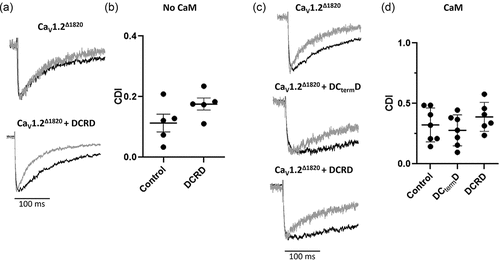
Figure 6. The DCRD peptide modify CaV1.2Δ1820 voltage-dependent inactivation: (a) Graph showing the residual of current after a 200-ms during a 250 ms depolarization pulse (R200, mean ± SEM. n = 8 for control and n = 7 for DCRD peptide) versus command voltage of L-type Ba2+ currents from AD293 cells overexpressing the CaV1.2Δ1820 channel with (empty circles) or without (filled circles) the DCRD peptide. Slower inactivation rates result in higher R200 values. (b) Graph shows the mean ± SEM of R200 from AD293 cells overexpressing the CaV1.2Δ1820 channel alone (n = 5), or with the DCtermD (n = 6) or the DCRD peptide (n = 5). (c) Graph shows the mean ± SEM of R200 from AD293 cells overexpressing calmodulin and the CaV1.2Δ1820 channel alone (n = 8), or with the DCtermD (n = 7), or the DCRD peptide (n = 6). The control data set of panel a and B is the same used in . *p < 0.05 with respect to control.
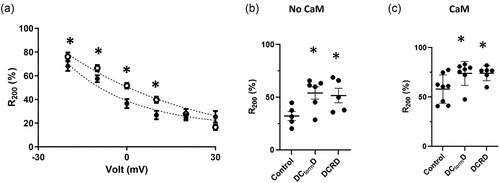
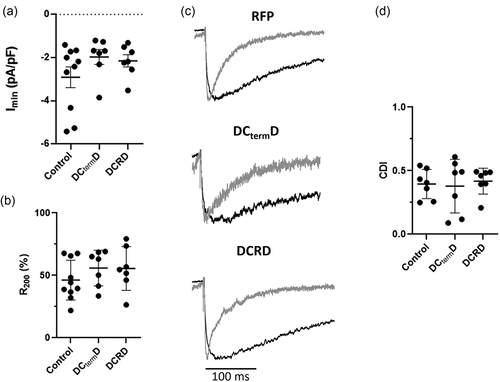
Figure 7. DCtermD and DCRD peptide effect over endogenous L-type currents in cardiomyocytes (a) Graph shows the mean ± SEM of the peak endogenous L-type Ca2+ current density from newborn rat cardiomyocytes (n = 10 for control, n = 7 for DCtermD, and n = 7 for DCRD peptide). (b) Graph shows the mean ± SEM of R200 newborn rat cardiomyocytes infected with RFP as control, or with the DCtermD-CFP, or the DCRD peptide coding sequence (n = 10 for control, n = 7 for DCtermD, and n = 7 for DCRD peptide). (c) Representative trace of the IBa (black line) and ICa evoked by a 0-mV depolarizing pulse. Both currents were scaled to the same amplitude for comparison. (d) Graph shows the mean ± SEM of CDI fraction (n = 7). *p < 0.05 with respect to control.
Supplemental Material
Download JPEG Image (273.3 KB)Supplemental Material
Download JPEG Image (388.6 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author, DV, upon reasonable request.
