Figures & data
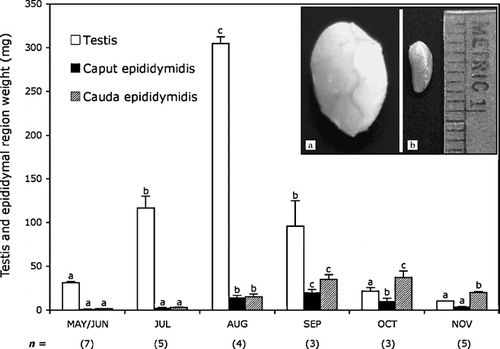
Figure 1 Changes in testicular and epididymal (caput and cauda) mass of adult male Corynorhinus mexicanus during the annual reproductive cycle. Bars indicate the mean±SE and the values in parentheses indicate the number of bats. Different letters indicate statistically significant differences (P < 0.05) between values in the same trace (ANOVA plus Bonferroni post-hoc test applied to the testis and epididymal segment data). The inserts show images of the macroscopical testes regression in Corynorhinus mexicanus: (a) testes of male bats captured on August 18, when maximal size is observed, and (b) testes of male bats captured on October 30, when full regression occurs. Metric units indicate millimeters.
Determination of the Number of Sperm Cells Recovered from Epididymal Regions at Selected Dates, and Percentage of Spermatozoa with a Cytoplasmic Droplet
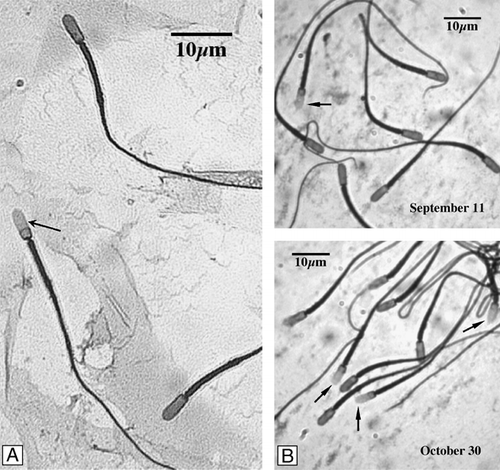
Figure 2 C. mexicanus spermatozoa. With cytoplasmic droplet (A, arrow). (B) Sperm cells exhibiting a high percentage with cytoplasmic droplet in the cauda epididymis (∼20%) in September that steadily decrease, reaching almost nil at the end of October.
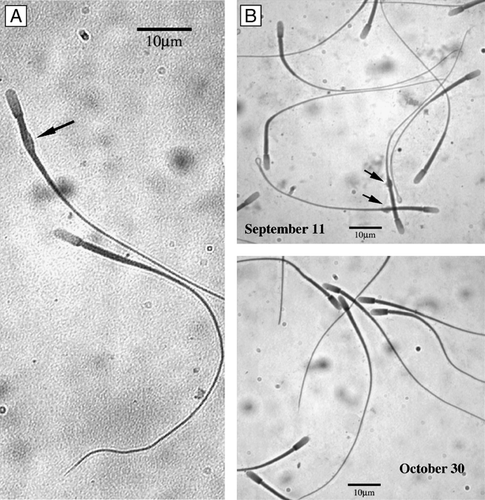
Figure 3 C. mexicanus spermatozoa capacitation. Spermatozoa were subject to the capacitation reaction during a 6 h incubation at 39°C under a 5% CO2/95% air atmosphere, in the presence of 2.5 mM Ca2+. Evaluation of the percentage of capacitated sperm was done by the chlortetracycline (CTC) fluorescence method. Percentage of capacitated sperm was observed at high magnification, using an epifluorescence Zeiss microscope with a 2Fl filter (405 nm excitation, 460 nm fluorescence and 510 nm barrier filters). (A) Presence of bright fluorescence in the acrosome region (arrow) was considered as providing evidence of capacitation. The neck region was always brilliantly stained by chlortetracycline. (B) Comparison between the epididymal cauda spermatozoa observed on September 11 and October 30, showing the increase in the percentages of capacitated sperm as the spermatozoa reside in the cauda epididymis.
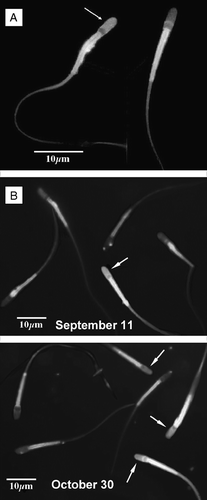
Figure 4 Acrosome reaction of C. mexicanus epididymal sperm. The acrosome reaction was assessed during a 60 min incubation, of previously capacitated spermatozoa, in the presence of 3.18 μM progesterone (final concentration). Afterwards aliquots were removed, fixed, and the percentage of acrosomally reacted spermatozoa determined by the coomasie brilliant blue method. Presence and number of stained and unstained spermatozoa was determined at high magnification. (A) Lack of blue staining of the acrosome region (arrow) indicates reacted acrosomes. Blue staining of the acrosome region forming a distinct apical ridge, indicates intact acrosomes of unreacted spermatozoa. (B) Comparison between the epididymal cauda spermatozoa observed in September 11 and October 30, showing the increase in the percentage of acrosome reacted sperm.
Percentage of Capacitated and Acrosome Reacted Spermatozoa Recovered from Different Epididymal Regions of C. mexicanus
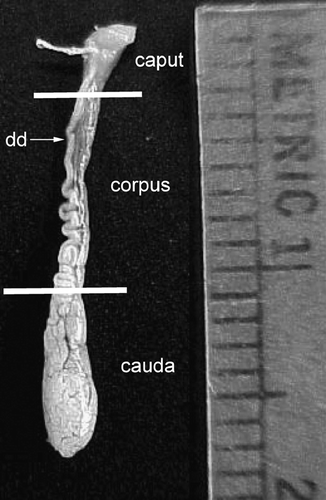
Figure 5 Fully developed epididymis of adult male Corynorhinus mexicanus. The caput, corpus and cauda regions are shown. Isolation of caput from the corpus was done by dissecting at the level at which the walls of the epididymis became clearly parallel (upper line). The cauda epididymis was isolated by dissecting at the site in which the deferens became clearly separated from the epididymis (lower line). Metric units indicate millimeters.