Figures & data
Figure 1. Immunofluorescence localization of galactosyl-alkyl-acyl-glycerol (GalAAG) in the testis of wild type (A,C) and twitcher mice (B,D) of tubules from stage VII-VIII. GalAAG (green) accumulation in Sertoli cell cytoplasm (asterisk) and spermatid tails (arrow head) is evident. Nuclei are stained in blue. Scale bar 25 μm.
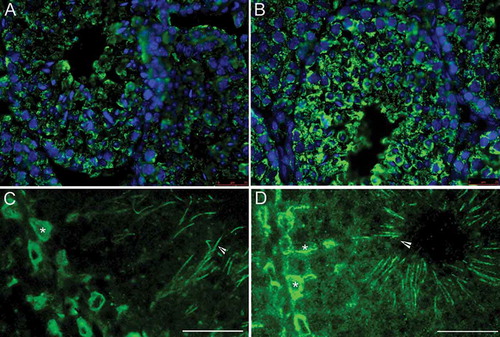
Figure 2. Light and transmission electron microscopy of twitcher and wild type seminiferous tubules at stage 4-5. Periodic acid-Schiff staining (PAS) highlights normal morphology of round spermatids both in wild type (A) and in twitcher (B). The acrosomes (arrow heads) are shown in red in the online version. Electron microscopy micrographs confirm that round spermatids at step 4-5 does not show differences between wild type (C) and twitcher (D); the acrosomal vesicle (asterisk) is tightly adherent to nuclear membrane and present a dense granule. BL: basal lamina; N: nucleus; M: mitochondria. Scale bar 20 µm (A,B), 2 µm (C,D).
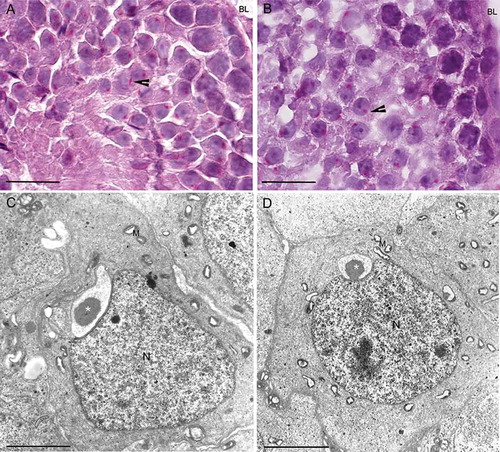
Figure 3. Light and transmission electron microscopy of twitcher and wild type tubule at stage 8. Periodic acid-Schiff staining (PAS) of round spermatids from wild type (A) and twitcher (B) mice shows an elongating acrosome cup-shaped. The acrosomes (asterisk) are shown in red in the online version. Electron microscopy micrographs showing spermatids from wild type (C) and twitcher mouse (D): the ruffling of the outer acrosomal membrane is evident only in gametes from mutant mice (arrowheads). BL: basal lamina; N: nucleus; M: mitochondria. Scale bar 100 µm (A,B), 3 µm (C,D).
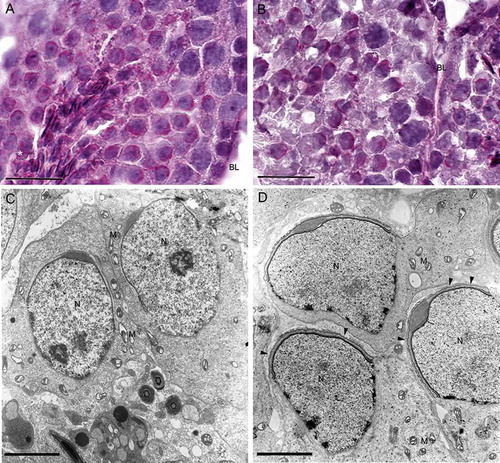
Figure 4. Light and transmission electron microscopy of twitcher and wild type tubule at stage 9. Periodic acid-Schiff staining (PAS) highlights the elongating acrosomes in wild type (A) and in twitcher (B), where altered acrosomal architecture is evident. The acrosomes (asterisk) are shown in red in the online version. Electron microscopy micrographs showing spermatids from wild type (C) and twitcher mouse (D). At ultrastructural level the sub-acrosomal space in twitcher spermatids appears enlarged (arrow heads) and swollen acrosomes and acrosomal projections in the anterior region of the nucleus are frequently detected (arrow). BL: basal lamina; N: nucleus; M: mitochondria. Scale bar 100 µm (A,B), 3 µm (C,D).
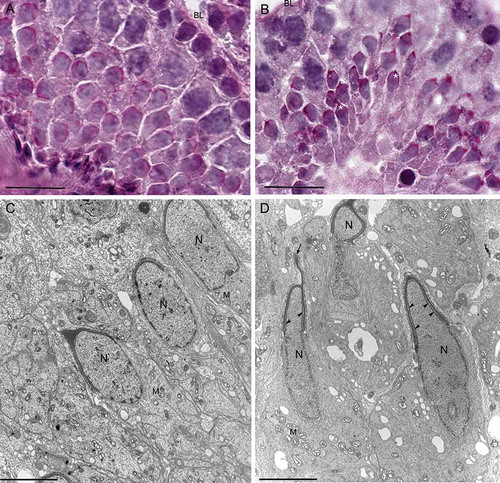
Figure 5. Electron microscopy micrographs of elongated spermatids at step 16 from wild type (A) and from twitcher mice (B). In wild type testis, spermatids have well conformed nucleus and acrosome. In panel B, the twitcher elongated spermatids reveal major structural acrosomal abnormalities. Indeed, the acrosome is redundant, swollen, and detached from the nucleus (arrow heads). N: nucleus; M: mitochondria. Scale bar 2 µm (A,B).
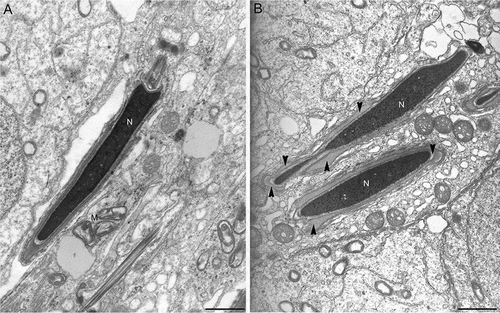
Figure 6. Transmission electron microscopy of corpus epididymidis from wild type (A,C) and from twitcher (B,D) mouse. The epithelium appears well structured both in wild type and in twitcher mice (A,B), but the apical region shows several differences: well shaped stereocilia are present in wild type mice (C) whereas, in twitcher mice, these structures are broken, detached, and completely spread out into the lumen (D). Sc: stereocilia. Scale bar: A,B, 10 µm; C,D, 2 µm.
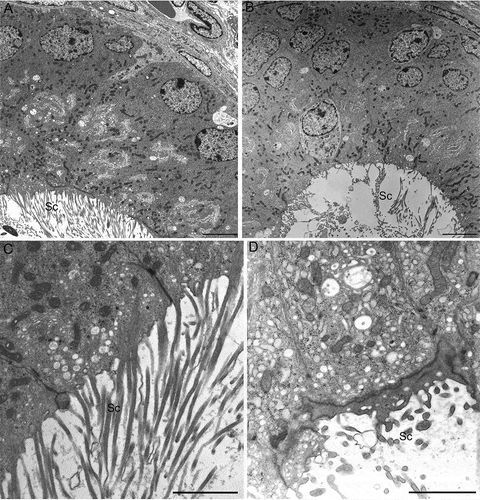
Figure 7. Transmission electron microscopy of sperm in the epididymal lumen at the level of cauda from wild type (A) and twitcher mouse (B,C). In twitcher epididymal lumen, misshaped sperms with structural acrosomal abnormalities (asterisk), large residual bodies, and cytoplasmic droplets are clearly visible. N: nucleus; Ax: axoneme; Sc: stereocilia. Scale bar 2 µm.
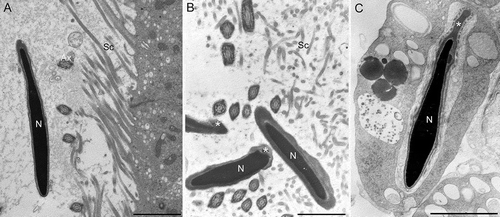
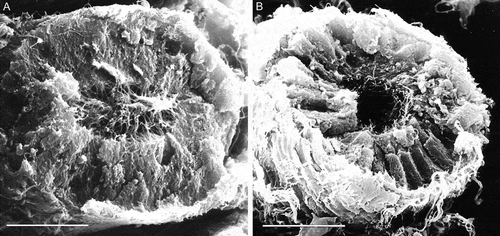
Figure 8. Scanning electron microscopy of epididymis from wild type (A) and twitcher mouse (B). In wild type mice well developed stereocilia project into the lumen of the tubule, while in the mutants the epididymal epithelium appears disorganized, the lumen larger and empty for the loss of stereocilia. Scale bar 20 µm.