Figures & data
Figure 1. Interaction of the BMP, TGFβ and GDF ligands to their respective receptors. (A) BMPs signaling via the SMAD protein dependent pathway in granulosa cells. BMP ligands initiate the signal transduction cascade by binding to type 1 receptor (BMPR1A/BMPR1B) and type II receptor (BMPRII) serine/threonine kinase and forming a heterotetrameric complex. The constitutively active type II receptor then transphosphorylates the type I receptor, and the type I receptor phosphorylates the Receptor-SMADs (either SMAD1/5/8). Phosphorylated SMAD1/5/8 associates with the co-SMAD (SMAD4), and the complex translocates into the nucleus where it further associates with co-activators or co-repressor to regulate the gene expression. (B) TGFβ signaling via the SMAD protein dependent pathway in granulosa cells. TGFβ1 ligand binding initiate the signal transduction cascade by binding to type 2 receptor (TGFβRII) and type 1 receptor (TGFβR1) and forming a heterotetrameric complex. The active type II receptor then transphosphorylates the type I receptor, and the type I receptor phosphorylates the receptor-SMADs (either SMAD2/3). Phosphorylated SMAD2/3 associates with the co-SMAD (SMAD4), and the complex translocates into the nucleus for regulate the expression of the target gene. (C) Growth and differentiation factor (GDF) signaling via the SMAD protein dependent pathway in granulosa cells. GDF9 ligand binding initiate the signal transduction cascade by binding to type 2 receptor (BMPRII) and type 1 receptor (TGFβR1) and forming a heterotetrameric complex. The active type II receptor then transphosphorylates the type I receptor, and the type I receptor phosphorylates the receptor-SMADs (either SMAD2/3). Phosphorylated SMAD2/3 associates with the co-SMAD (SMAD4), and the complex translocates into the nucleus where it bind to the regulatory sequences and regulate the expression of the target gene.
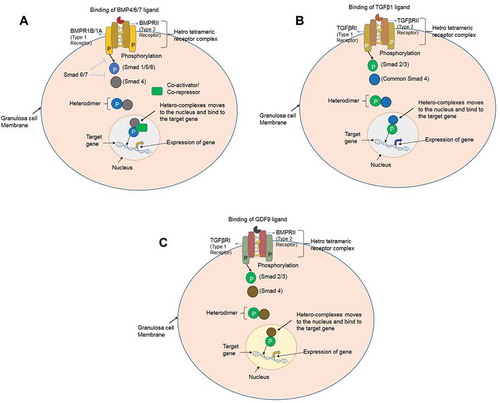
Figure 2. Aspiration and culture of the granulosa cells from antral follicle of sheep. Antral follicles (~3.5mm) were punctured by 26G needle (Becton Dickenson) and the follicular fluid was aspirated for culturing of the sheep granulosa cells. The morphological features of the granulosa cells of non-carrier Malpura (FecB++) (X), homozygous carrier GMM (FecBBB) (Y), and non-carrier GMM (FecB++) (Z) shown in the culture of McCoy’s 5A medium. All granulosa cells were healthy and appeared like an epithelial cells.
Figure 3. Expression of the BMP/SMAD family of genes in sheep granulosa cells by reverse transcription-polymerase chain reaction (RT-PCR). Representative agarose gel photograph showing the amplification of BMP factors/receptors and SMAD signaling genes. M: 50bp DNA ladder (Fermentas), Lane 1: RPL19, Lane 2: SMAD1, Lane 3: SMAD 3, Lane 4: SMAD 4, Lane 5: SMAD 5, Lane 6: SMAD 8, Lane 7: ACVR2, Lane 8: CYP11A1, Lane 9: AMH, Lane 10: BMPR1B, Lane 11: BMP15.
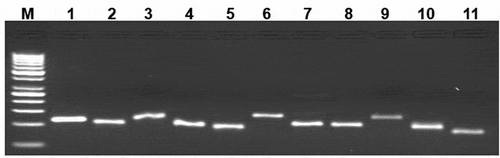
Figure 4. Quantitative expression of the BMP factors/receptors in the granulosa cells of different FecB genotypic groups of ewes. Relative abundance (RFU, relative fold unit) of the BMP factors and BMP receptors shown in granulosa cells of homozygous carrier GMM (FecBBB), non-carrier GMM (FecB++), and non-carrier Malpura (FecB++) ewes with reference to RPL19 as housekeeping gene (internal control). Pairwise significant difference between means of different FecB groups was considered from P value lower than 0.05 to 0.001. Straight lines between two values with asterisks above them depict the significant difference as *P < 0.05, **P < 0.01, ***P < 0.001.
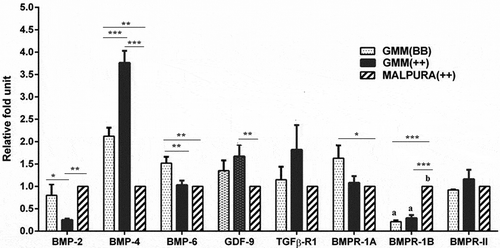
Figure 5. Quantitative expression of the SMAD signaling genes in the granulosa cells of different FecB genotypic groups of ewes. The relative abundance (RFU, relative fold unit) of the SMAD signaling genes shown in the granulosa cells of homozygous carrier GMM (FecBBB), non-carrier GMM (FecB++) and non-carrier Malpura (FecB++) ewes with reference to RPL19 as housekeeping gene (internal control). Pairwise significant difference between means of FecB groups was considered from P value lower than 0.05 to 0.001. No significant difference between means of gene expression data (RFU) of different FecB groups was recorded.
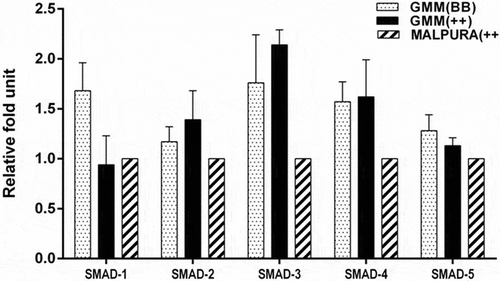
Figure 6. Quantitative expression of the hormone, hormone receptor and steroidogenic associated genes in the granulosa cells of different FecB genotypic groups of ewes. Relative abundance of the hormone, hormone receptor and steroidogenic associated genes in GCs of homozygous carrier GMM (FecBBB), non-carrier GMM (FecB++) and non-carrier Malpura (FecB++) ewes with reference to RPL19 as housekeeping gene (internal control). Pairwise significant difference between means was considered from P value lower than 0.05 to 0.001. Straight lines between two values with asterisks above them depict the significant difference as *P < 0.05, **P < 0.01, ***P < 0.001.
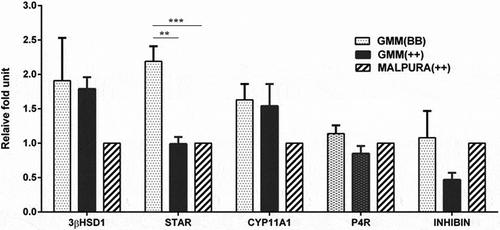
Figure 7. Schematic representation of the BMP factors/receptors, SMAD signaling molecules, and steroidogenesis associated genes in granulosa cells. Binding and interactions of the ligand molecules viz. BMP2/4/6/7, GDF9 and TGFβ1 to their respective receptors molecules BMPRII/TGFβRII. Phosphorylation of the receptor regulated SMAD proteins (either SMAD1/-2/-3/-5/-8) and their association with common SMAD4. Entry of the hetero-complexes into nucleus and acting as transcription activators for expression of the target genes of a particular pathway. Secretion of the progesterone and inhibin after activation through their receptors. Depiction of the expression of the steroidogenesis associated genes in the granulosa cells.
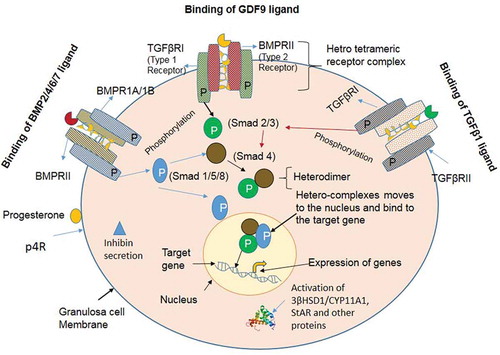
Table 1. List of the primer sequences and their expected product size (base pair).
