Figures & data
Table 1. Oxidative damages to the molecular targets of reactive oxygen species.
Figure 1. Chemical structures of arachidonic acid and F2-isoprostane series. Isoprostanes are named based upon the structure of the functional groups on the cyclopentane ring in a manner analogous to that of prostaglandins. F2-isoprostanes (F2-IsoPs) are F2-prostaglandin-like compounds formed from the free radical-catalyzed peroxidation of arachidonic acid (ARA). Based on the mechanism of formation, four F2-IsoP series are generated. The four F2-IsoP series are named (5, 8, 12, and 15 series) according to the carbon number on which the side chain hydroxyl group is attached, carboxyl carbon being 1. In the figure, chemical structure of ARA and F2-IsoP series are shown.
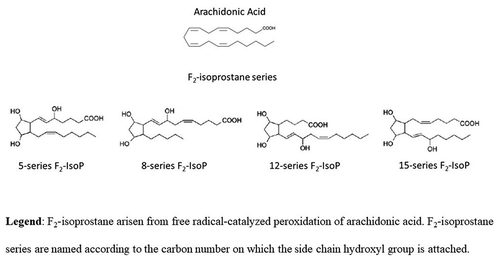
Figure 2. Synthesis of F2-isoprostanes from arachidonic acid esterified in membrane phospholipids. In contrast to cyclooxygenase-derived prostaglandin that are generated from free arachidonic acid (ARA), F2-isoprostanes (F2-IsoPs) are initially formed from the oxidation (free-radical-induced peroxidation) of ARA esterified in membrane phospholipids (in the figure, membrane phospholipid bilayer with esterified ARA and F2-IsoPs are shown). F2-IsoPs can then be released from the phospholipid backbone as free fatty acids (free F2-IsoPs in the figure) by phospholipase action (Phospholipase A2 activity, in the figure).
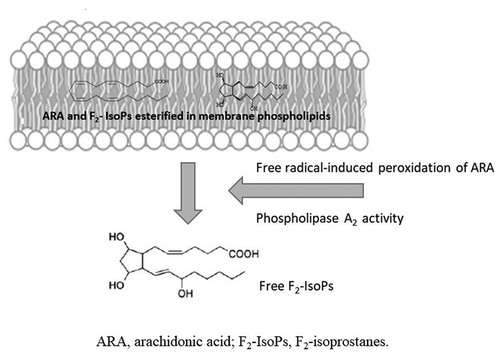
Figure 3. F2-IsoP formation is valuable in both spermatozoa and seminal plasma. Upon semen fractionation (by centrifugation) into its seminal plasma and spermatozoa, F2-isoprostanes (F2-IsoPs) levels were determined, as free F2-IsoPs and esterified F2-IsoPs, respectively. According to the mechanism shown in and the text, in seminal plasma free F2-IsoPs can be released from the phospholipid backbone of sperm (or other different cell) membrane; in spermatozoa, F2-IsoPs can be detected as still esterified to phospholipid.
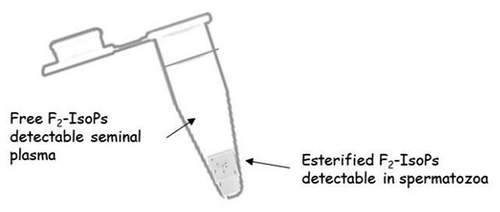
Table 2. Current relevant findings on isoprostanes in male infertility.
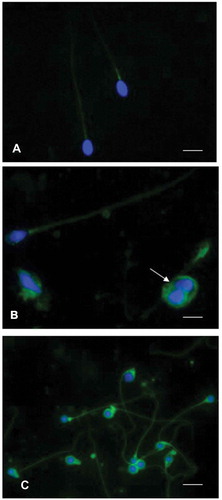
Figure 4. Immunofluorescence staining of spermatozoa using an anti8-iso-PGF2α polyclonal antibody and 4,6‐Diamidino‐2‐phenylindole (DAPI) solution. (A) sperm from an idiopathic infertile patient show a weak label of anti8-iso-PGF2α in the sperm tail, the signal appears more evident at the level of the mitochondrial sheath. The nuclei are staining with DAPI. In (B), showing spermatozoa from an infertile varicocele patient, the anti8-iso-PGF2α staining is located in the tail and in presence of cytoplasmic droplets (arrow). The nuclei stained with DAPI are altered. In (C), spermatozoa of a globozoospermic patient are shown; the nuclei appear perfectly round as evidentiated by DAPI, the anti8-iso-PGF2α fluorescent label is strongly evident in the tails, sometimes coiled, and in the abundant cytoplasmic residues surrounding the round heads. A, B: bar 5 µm, C: bar 7 µm.