Figures & data
Figure 1. Photomicrographs at low magnification of human testicular biopsies. A) Bouin/paraffin (B/P) processing followed by hematoxylin & eosin staining. B) Glutaraldehyde/glycol methacrylate (G/GMA) processing followed by toluidine blue-sodium borate staining. Observe in the B/P processed sample (A) the reduced diameter of the seminiferous tubules as well as a poor morphological distinction between the different types of germ cells of the seminiferous epithelium (Ep) and the components of the interstitium (In) when compared to the processing with G/GMA (B). Lu: lumen; TP: tunica propria; BV: blood vessel. Bars: 50 μm
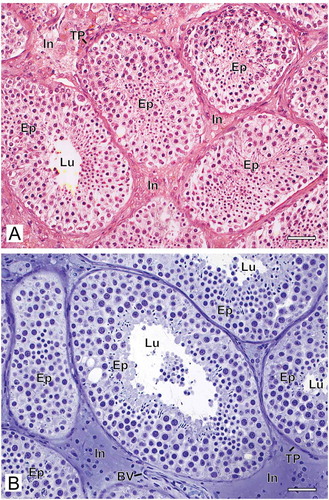
Figure 2. Photomicrographs at higher magnification of human testicular biopsies. A,C,E,G) Glutaraldehyde/glycol methacrylate (G/GMA) processing followed by toluidine blue-sodium borate staining. B,D,F,H) Bouin/paraffin (B/P) processing followed by hematoxylin & eosin staining. After G/GMA (A), germ cells (Adark and Apale spermatogonia; P: pachytene spermatocyte; R: round spermatid; E: elongating spermatid) and somatic cells (S: Sertoli cell; My: myoid cell; Mc: mast cell) as well apoptosis (Ap) and tunica propria (TP) were promptly recognized. After B/P (B), it is difficult to confidently recognize different spermatogonial subtypes (Sg). Lu: lumen. The processes of mitosis (C,D) and apoptosis (E,F) were more easily recognized and the initial hyalinization of tunica propria (C,E, arrows) after G/GMA. After G/GMA (G), Leydig cells showed evident round nucleus and nucleolus and the cytoplasm was either less evident (L1) or full of lipid droplets (L2). These morphological details were not observed after processing with B/P (H). ST: seminiferous tubule; BV: blood vessel. Bars: A-B, 23 μm; C-D’, 10 μm; E-F, 15 μm
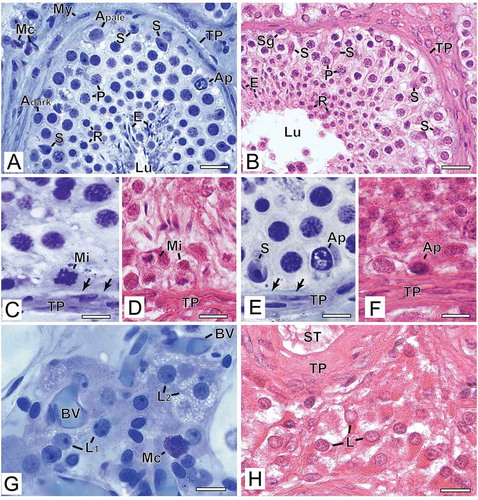
Figure 3. Photomicrographs illustrating changes in spermatogenesis scoring. (A-E) Bouin/paraffin (B/P) processing followed by hematoxylin & eosin staining. (A’-E’) Glutaraldehyde/glycol methacrylate (G/GMA) processing followed by toluidine blue-sodium borate staining. The arrows show diagnostic changes that occurred when samples initially scored with B/P were reevaluated with G/GMA. Numbers inside the circles represent the number of cases with diagnostic change after rescoring. P: pachytene spermatocyte; E: elongating spermatid; S: Sertoli cell; Fb: fibroblast. Bar: 50 μm
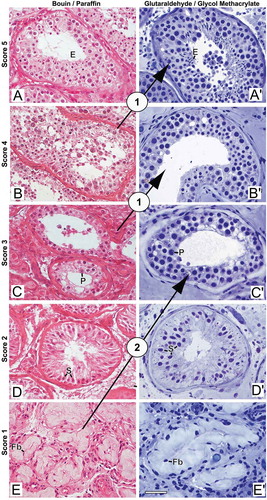
Figure 4. Quantitative Results. (A) Spermatogenesis scores assigned to paired fragments of testicular biopsies (n = 21) processed with Bouin/paraffin (B/P, yellow bars) or glutaraldehyde/glycol methacrylate (G/GMA, blue bars). The cases are divided according to the recovery of sperm for ICSI. (B) Receiver operating characteristics (ROC) curves of spermatogenesis score to predict sperm recovery for ICSI. The curves represent the scores assigned to biopsies processed by B/P or by G/GMA
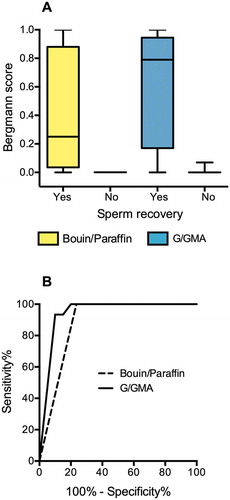
Table 1. Clinical characteristics of the study participants
