Figures & data
Table 1. Characteristics of the patients at baseline
Table 2. Estimated pharmacokinetic and pharmacokinetic-pharmacodynamic parameters
Figure 1. Observed and model-predicted cardiac index (CI) over time in patients. Closed circles are observed CI in patients treated with 6 injections de 5 mg/kg bevacizumab every other week. Continuous line and shaded areas represent median and 5th, 25th, 75th and 95th percentiles of model-predicted CI.
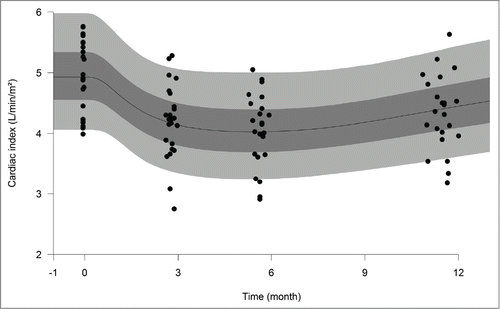
Figure 2. Observed frequencies and model-predicted probabilities of epistaxis daily durations. White, gray and dark gray bars are ‘no epistaxis’, ‘less than or equal to 10 min’ and ‘11 to 20 minutes’ mean daily epistaxis observed frequencies, respectively. Solid, dashed and long-dashed lines are the model predicted probabilities for ‘no epistaxis’, ‘less than or equal to 10 min’ and ‘less than or equal to 20 min’ daily epistaxis, respectively.
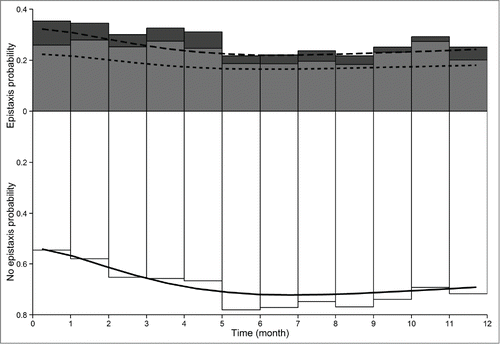
Figure 3. Simulations of bevacizumab concentrations and effects obtained with 4 different maintenance dosing regimen over 3 years. DR1 consists in 6 injections of 5 mg/kg bevacizumab, every over week, every year (3 upper panels). DR2, DR3 and DR4 consist in 6 injections of 5 mg/kg bevacizumab, every over week, followed by injections of 5 mg/kg every 3, 2 and 1 months, respectively (lower panels). Solid lines and shaded areas are median and 5th-95th percentiles of simulated bevacizumab concentrations (left panels), simulated cardiac index (center panel) and simulated epistaxis duration (right panel).
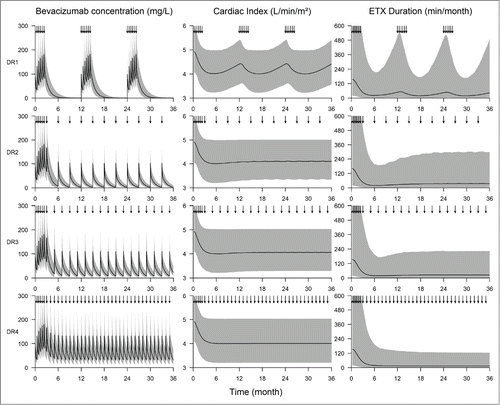
Figure 4. Simulation of proportion of responders at 24th and 30th month according to bevacizumab maintenance dosing regimen. DR1 consists in 6 injections of 5 mg/kg bevacizumab, every other week, every year. DR2, DR3 and DR4 consist in 6 injections of 5 mg/kg bevacizumab, every other week, followed by injections of 5 mg/kg every 3, 2 and 1 months, respectively. Each bar is divided into 4 shades of gray according to quartiles of the initial values of cardiac index and of the initial epistaxis duration. Clearest gray is the first quartile (mildest symptoms) and darkest is the fourth (highest severity of symptoms).
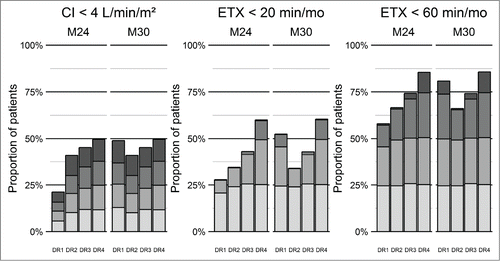
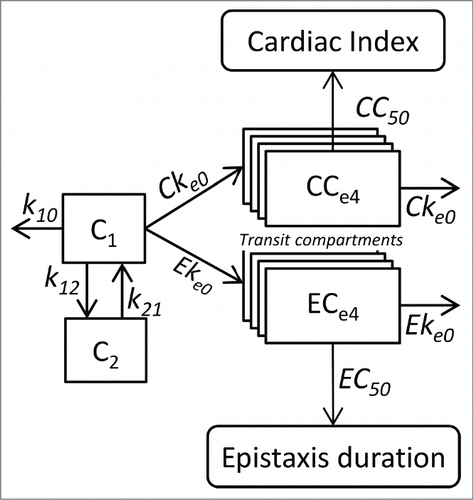
Figure 5. Pharmacokinetic and pharmacokinetic-pharmacodynamic models relating cardiac index and epistaxis duration with bevacizumab concentrations. Bevacizumab pharmacokinetics are described using a 2-compartment model with linear elimination. Effects of bevacizumab are described using transit effect-compartments. The concentrations in the last effect-compartments influence cardiac index and risk of epistaxis through sigmoid Emax models. C1 and C2 are concentrations in the central and peripheral compartments, respectively. k10 is elimination rate and k12 and k21 are distribution rates. CCe1 to CCe4 are transit effect-compartment concentrations. Cke0 is transit rate constant. CI0 and CImin are initial and minimal cardiac index, respectively. CC50 is CCe4 concentration leading to a cardiac index of (CI0+CImin)/2. ECe1 to ECe4 are transit effect-compartment concentrations. Eke0 is the transit rate constant. λ0 is the Poisson distribution parameter of the epistaxis duration classes. EC50 is ECe4 concentration leading to λ = λ/2.