Figures & data
Figure 1. Bortezomib and IFNα fusion proteins act synergistically in inhibiting proliferation in HMCLs. HMCLs were treated for 3 days with fusion protein or bortezomib. Percent proliferation was calculated relative to cells grown in the absence of treatment by MTS. Each cell line was examined 3 to 5 times. Representative experiments are shown with data presented as the mean of triplicate experiments with the error bars indicating the SEM. (A) OCI-My5, NCI-H929, and ANBL-6 cells were treated with 1.25 – 10 nM bortezomib or 1.25 – 10 pM anti-CD138-IFNα14. U266 cells were treated with 0.4 – 50 nM bortezomib or 0.4 – 50 pM anti-CD138-IFNα14. (B) Cells were treated with anti-CD138-IFNα2, anti-CD138-IFNα2YNS, or anti-CD138-IFNα14 in the presence or absence of bortezomib. The concentrations used were 3 pM fusion protein and 1 nM bortezomib for OCI-My5; 5 pM fusion protein and 5 nM bortezomib for NCI-H929 and ANBL-6; 1.5 pM anti-CD138-IFNα2, 0.5 pM anti-CD138-IFNα2YNS, 1.5 pM anti-CD138-IFNα14, and 1.5 nM bortezomib for U266. Panels A and B are from independent experiments.
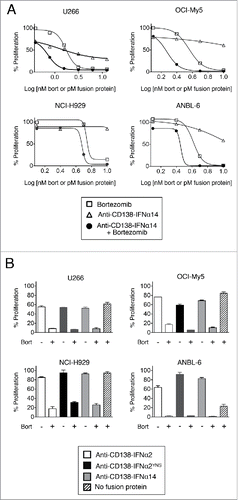
Table 1. Quantitative determination of drug interactions between bortezomib and IFNα fusion proteins.
Figure 2. Activation of STAT1 in cells treated with bortezomib or anti-CD138-IFNα14. Cells were treated with anti-CD138-IFNα14, bortezomib or both for 0.5, 24 and 48 h. NCI-H929 was treated with 2.5 pM fusion protein or 5 nM bortezomib. OCI-My5 were treated with 3 pM anti-CD138-IFNα14 or 1.5 nM bortezomib. Total STAT1 protein and pSTAT1 levels in cell lysates were determined by Western blotting. Protein loading was monitored by probing the same membranes with anti-GAPDH.
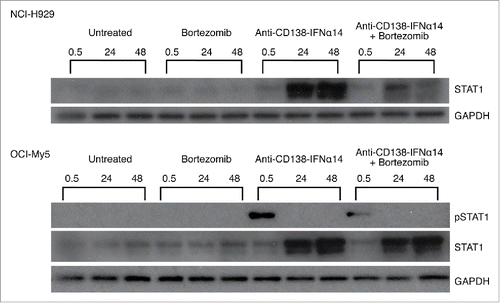
Figure 3. IRF4 expression is down-regulated by bortezomib + anti-CD138-IFNα14 treatment. Cells were treated with anti-CD138-IFNα14, bortezomib or both for 24 and 48 h. U266 and OCI-My5 were treated with 3 pM anti-CD138-IFNα14 or 1.5 nM bortezomib. ANBL-6 was treated with 6 pM fusion protein or 2.3 nM bortezomib. NCI-H929 was treated with 2.5 pM fusion protein or 5 nM bortezomib. The levels of IRF4 in cell lysates were determined by Western blotting. IRF4 levels were quantitated using NIH ImageJ and expressed as a percentage of the untreated level as described in Materials and Methods.
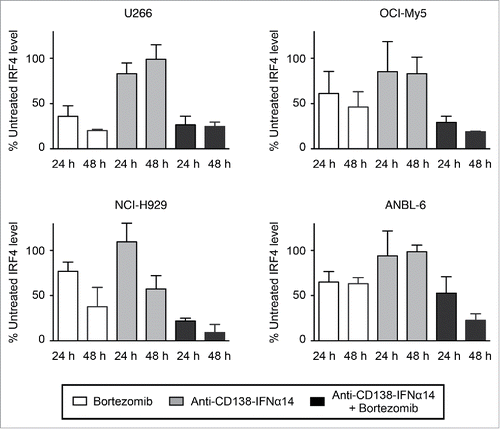
Figure 4. Induction of cell death is not prevented by caspase inhibition. Cells were treated with anti-CD138-IFNα14 and bortezomib in the presence or absence of pan-caspase inhibitors for 3 days. U266 and OCI-My5 were treated with 3 pM anti-CD138-IFNα14 and 1.5 nM bortezomib. NCI-H929 and ANBL-6 were treated with 5 pM anti-CD138-IFNα14 and 4 nM bortezomib. Cells were assessed for changes in metabolic activity by MTS. Assays were done in triplicate with the error bars indicating the SEM. In some cases, the error bars cannot be seen because they are obscured by the plot. Percent proliferation was calculated relative to cells grown in the absence of treatment. (A) 50 μM z-VAD-fmk or 20 μM Q-VD-OPh. (B) 20 μM z-FA-fmk.
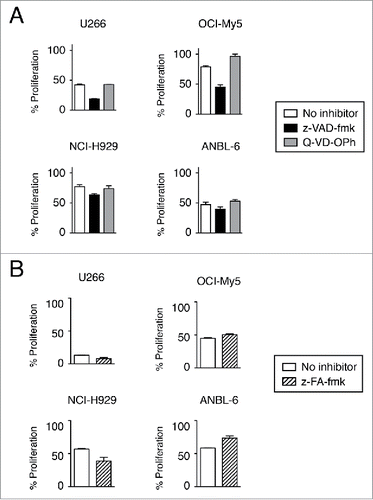
Figure 5. ROS generation plays a role in the induction of cell death by bortezomib + anti-CD138-IFNα14. Cells were treated for 3 days with anti-CD138-IFNα14 or bortezomib in the presence or absence of 1 mM tiron to inhibit ROS. U266 and OCI-My5 were treated with 3 pM anti-CD138-IFNα14 or 1 nM bortezomib. NCI-H929 and ANBL-6 were treated with 5 pM anti-CD138-IFNα14 or 4 nM bortezomib. Cells were stained with Alexa Fluor 488-labeled Annexin V and PI and analyzed by flow cytometry to assess the level of apoptosis.
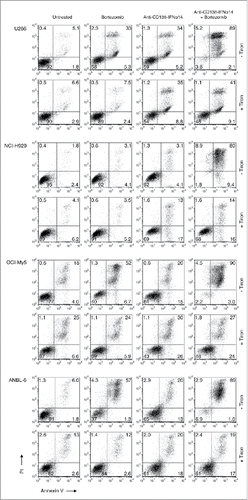
Figure 6. The effect of bortezomib or anti-CD138-IFNα14 treatment on mitochondrial dysfunction. (A) Cells were treated with anti-CD138-IFNα14 or bortezomib for 2 days. The concentrations of anti-CD138-IFNα14 and bortezomib were: U266 (3 pM, 1 nM), NCI-H929 (5 pM, 3 nM), ANBL-6 (5 pM, 3.5 nM), and OCI-My5 (3 pM, 1.5 nM). After treatment, cells were incubated with DiOC6(3) and mitochondrial membrane potential ΔΨm measured by flow cytometry. (B) ANBL-6 and U266 cells were untreated (lane 1) or treated for 24 h with anti-CD138-IFNα14 (lane 2), bortezomib (lane 3), or both (lane 4). Western blot analysis of cell lysates was used to determine the levels of uncleaved and cleaved PARP. (C) OCI-My5 and NCI-H929 cells were treated for 0.5, 24 or 48 h with anti-CD138-IFNα14, bortezomib, or both and the levels of uncleaved and cleaved PARP were determined by Western blotting. For (B) and (C), protein loading was monitored by probing the same membranes with anti-GAPDH. PARP – full length. PARP* – cleaved.
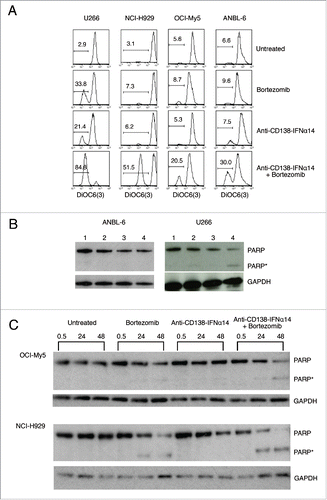
Figure 7. Bortezomib and anti-CD138-IFNα14 act synergistically to induce apoptosis in HMCLs. HMCLs were treated with anti-CD138-IFNα14 or bortezomib for 3 days. U266 and OCI-My5 were treated with 3 pM anti-CD138-IFNα14 or 1 nM bortezomib. NCI-H929 and ANBL-6 were treated with 5 pM anti-CD138-IFNα14 or 4 nM bortezomib. Cells were stained with Alexa Fluor 488-labeled Annexin V and PI and analyzed by flow cytometry to assess the level of apoptosis.
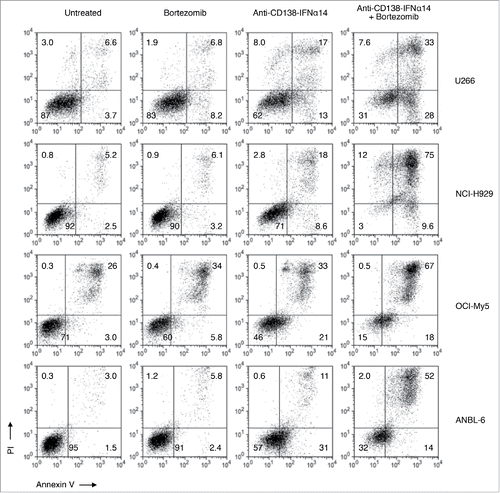
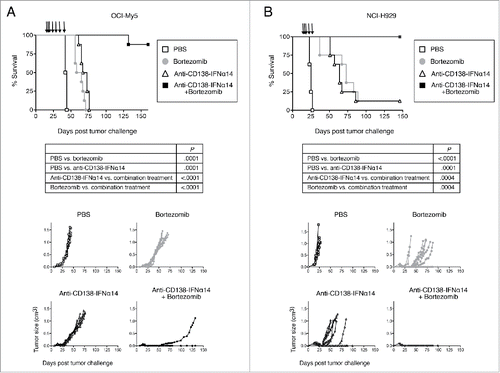
Figure 8. Synergistic protection against tumor growth is observed between anti-CD138-IFNα14 and bortezomib in vivo in murine models of MM. (A) OCI-My5 tumors were established in SCID mice by subcutaneous injection of cells. Mice were treated iv on days 14, 16, 18, and then ip on days 21, 28, 35 and 42 for a total of 7 treatments. (B) NCI-H929 tumors were established in NSG mice by subcutaneous injection of cells. Mice were treated iv on days 14, 16, 18 and then ip on days 21 and 28 for a total of 5 treatments. For both models, each group consisted of 8 mice. Treatment groups included PBS, 100 µg anti-CD138-IFNα14, 0.75 mg/kg bortezomib or 100 µg anti-CD138-IFNα14 + 0.75 mg/kg bortezomib. Tumor growth and survival were monitored.