Figures & data
Table 1. Quality attributes of a panel of FDA/EMA approved and clinical stage mAb products.
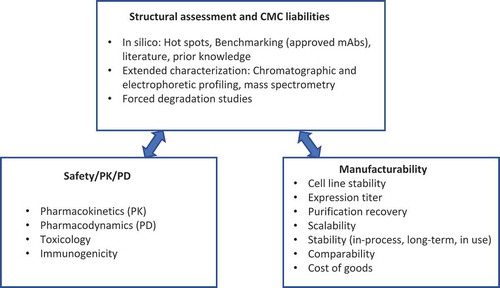
Table 2. Categories of mAb attributes.
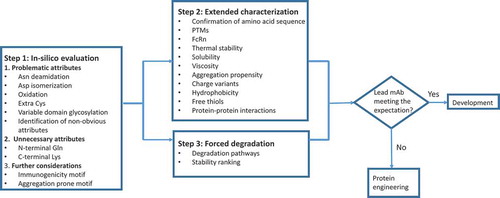
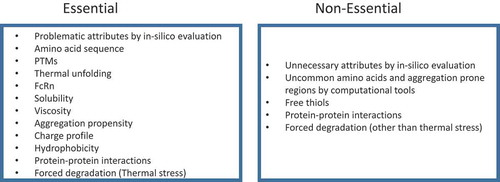
Table 3. mAb quality attributes evaluated during developability assessment.
Table 4. Known PTMs of mAbs identified by LC-MS.
Table 5. Modifications that form either acidic or basic species.
Table 6. Modifications causing HIC retention time shift.
Table 7. Forced degradation conditions, degradation pathways, and recommendations for developability evaluation.