Figures & data
Table 1. Monoclonal antibodies undergoing late-stage clinical studies or authorized for COVID-19*
Table 2. Antibody therapeutics granted first approvals in the European Union or the United States during 2020*
Figure 1. Number of antibody therapeutics first approved in the United States or European Union each year during 1997–2020*
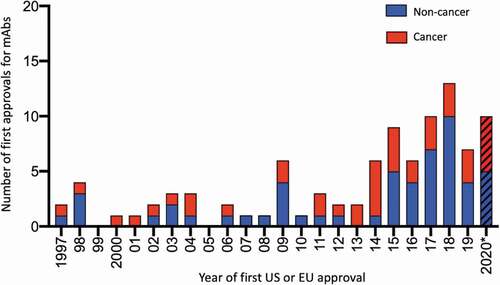
Table 3. Investigational antibody therapeutics in regulatory review in the European Union or the United States*
Table 4. Investigational monoclonal antibodies in late-stage clinical studies for non-cancer indications*
Table 5. Investigational monoclonal antibodies in late-stage clinical studies for cancer indications*
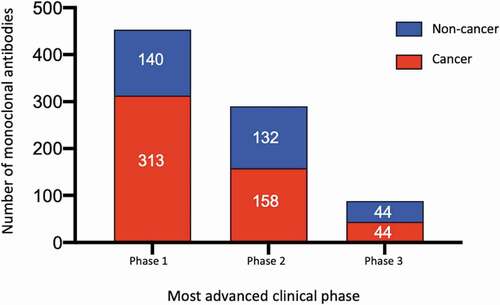
Figure 2. Global commercial clinical pipeline of monoclonal antibodies*