Figures & data
Figure 1. Structural analysis of HC-LC interface
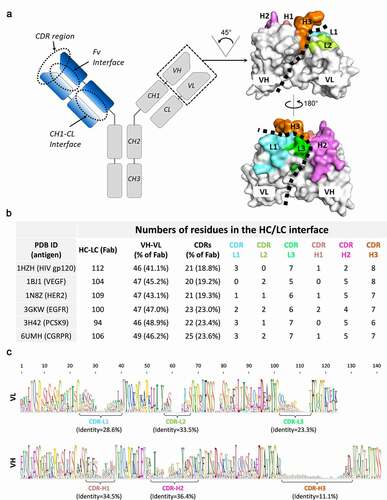
Figure 2. Schematic representation of the design of high-throughput Chain Selective Assessment methods (CSA)
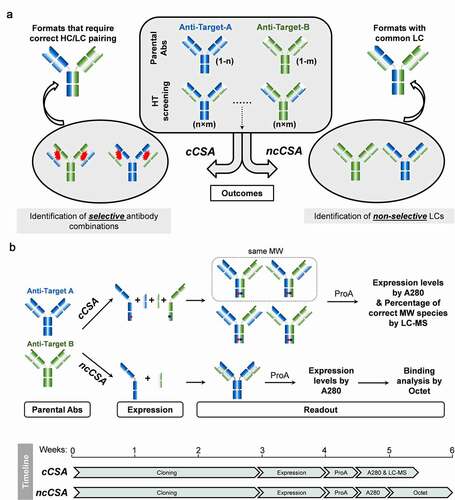
Figure 3. High-throughput screening for low cross-pairing antibody combinations in cCSA experiment
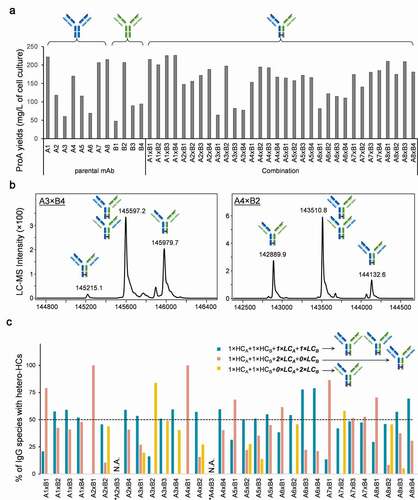
Figure 4. Predictability of HC/LC pairing in 4-chain hetero-IgGs
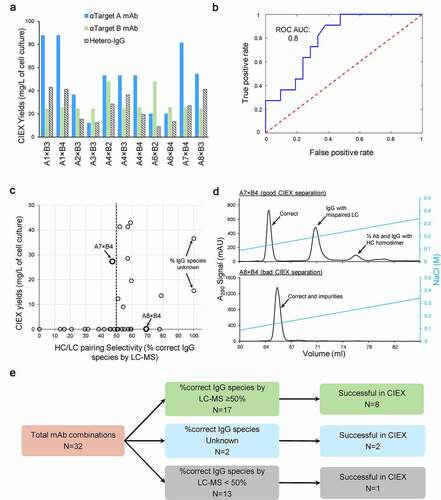
Figure 5. High-throughput screening for cLC hetero-IgGs by ncCSA
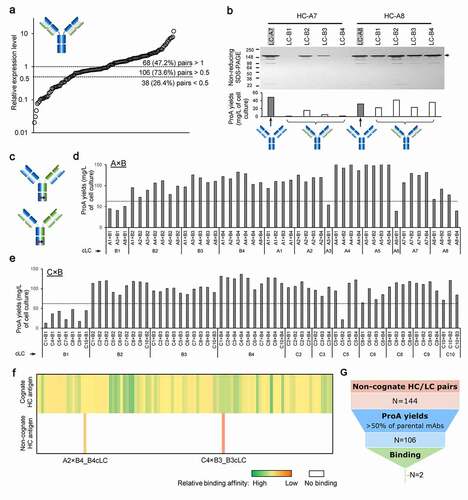
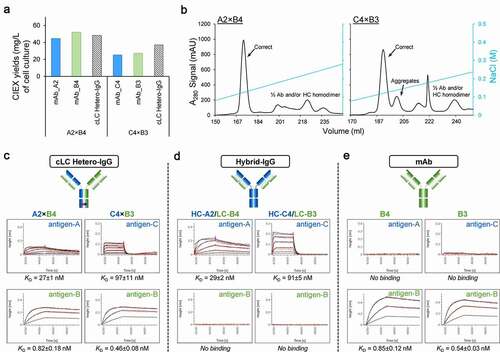
Figure 6. Expression, purification and binding properties of two selected cLC hetero-IgG molecules