Figures & data
Table 1. SEC-MALS of CD96 and CD155 proteins and complexes
Table 2. SPR-binding kinetics and ligand blocking activity of anti-mouse CD96 antibodies
Figure 1. Binding and ligand blocking of anti-mouse CD96 bivalent (filled symbols) and monovalent (open symbols) IgGs on cells. (a) Binding of antibodies to mouse CD96-expressing CHO cells (mean ± SD, n = 3; independent experiments performed three times). (b) Antibody blocking of mouse CD155 tetramers (mean ± SD, n = 2, independent experiments performed three times). All antibodies were expressed in the mIgG1-D265A isotype
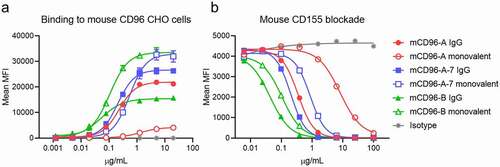
Table 3. SEC-MALS of antibody-CD96 complexes. The individual Fab and IgG molecular weights are approximately 48 kDa and 143 kDa, respectively
Figure 2. Cartoon schematic of binding assemblies of CD155 ligand, Fabs, and IgGs to mouse CD96 reveals two distinct antibody blocking mechanisms
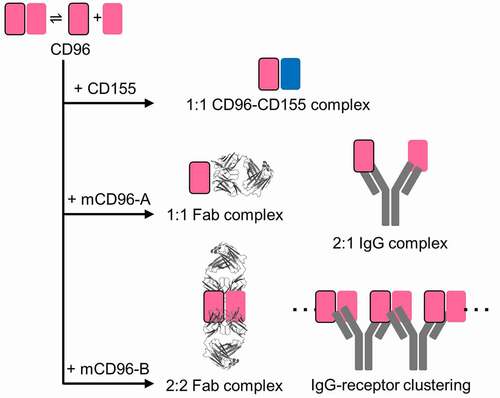
Figure 3. Antibodies mCD96-A and mCD96-B bind distinct epitopes on monomeric or dimeric mouse CD96 to block CD155. The crystal structures of CD96 in complex with (a) CD155 ligand, (b) mCD96-A Fab, and (c) mCD96-B Fab. The complexes are aligned onto CD96, represented as a light gray surface. In the mCD96-B structure, the other protomer of CD96 that forms the dimer is shown as a dark gray surface. CD155, mCD96-A, and mCD96-B are colored as blue, red, or green cartoons, respectively. The CD96-CD155 complex is of the human orthologs from PDB ID 6ARQ. (d) Epitopes of CD155, mCD96-A, and the CD96 dimer interface are colored blue, red, or dark gray on the surface representation of CD96. Each CD96 protomer is aligned in the same orientation. Regions contacted by the mCD96-A heavy chain or light chain are colored red or light red, respectively. (e) Epitope of mCD96-B on the surface of the CD96 homodimer, with each protomer colored light or dark gray. Regions contacted by the mCD96-B heavy chain or light chain are colored green or light green, respectively
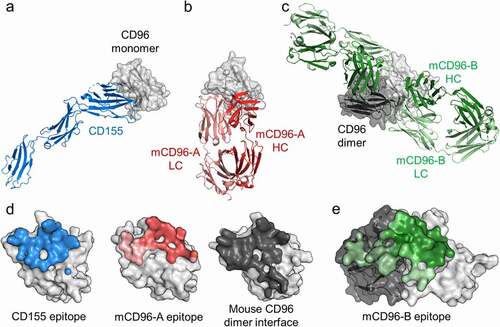
Figure 4. Comparisons of the human and mouse CD96 D1 domains. (a) Structural alignment of the D1 domains of the human CD96-CD155 heterodimeric complex (PDB ID 6ARQ) and the mouse CD96 homodimer from the mCD96-B complex. (b) Sequence alignment of the human and mouse CD96 D1 domains. Interacting residues to human CD96 by CD155 or of the mouse CD96 homodimer conserved across both protomers were calculated by Areaimol and are highlighted blue or gray, respectively

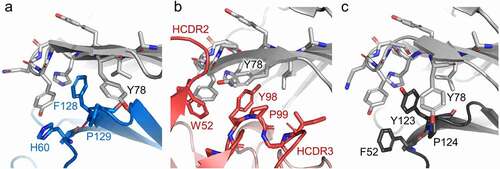
Figure 5. CD96 molecular recognition similarities at the lock-and-key interface. The (a) CD155, (b) mCD96-A, (c) and mouse CD96 dimer complexes are represented as cartoons and the residues of the ligand, mCD96-A, and opposing CD96 protomer that form the key are shown as sticks and are colored blue, red, or dark gray, respectively. The CD96 lock and Y78 residues in each of the complexes are shown as sticks and are colored light gray