Figures & data
Table 1. Overview of developed therapeutic antibody-encoding OVs: OVs expressing recombinant IgGs/monoclonal antibodies (Viro-MAb therapy) sorted according to targets and publication date
Table 2. Overview of developed therapeutic antibody-encoding OVs: OVs expressing BiTEs (viro-BiTE therapy), sorted according to target and publication date
Table 3. Overview of developed therapeutic antibody-encoding OVs: OVs expressing immune checkpoint inhibitors (viro-CHECKin therapy), sorted according to target and publication date
Table 4. Overview of developed therapeutic antibody-encoding OVs: OVs expressing anti-angiogenic antibodies (viro-ANGin therapy), sorted according to target and publication date
Table 5. Overview of the developed therapeutic antibody-encoding OVs: OVs expressing cytotoxic immunofusions (viro-iTOX therapy)
Figure 1. Viro-antibody therapy – an overview. Transgenes encoding recombinant therapeutic antibodies are inserted into the genome of oncolytic viruses (OVs) by exploitation of diverse strategies for transgene expression (see ). OV-encoded antibodies are produced locally in the tumor by infected cancer cells and expression lasts as long as active OV infection and spread is ongoing. The produced antibodies, dependent on their format, valency and size (see ), perfuse the tumor, bind their target on (noninfected) cancer cells or cancer-associated cells, and trigger their direct or indirect killing via diverse modes of action (see )
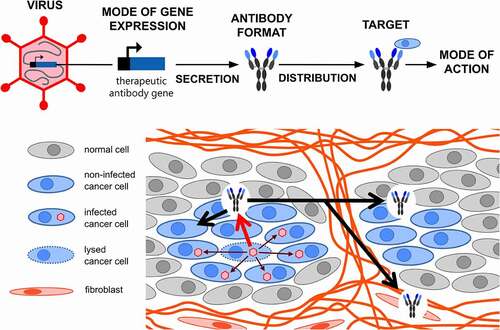
Figure 2. Viruses and modes of antibody expression exploited for viro-antibody therapy. A panel of viruses with or without envelope and with DNA (double-strand) or RNA genomes (negative strand, continuous for MeV, NDV, VSV or segmented for influenza virus) have been exploited for viro-antibody therapy (left panel). Dependent on the virus chosen, different strategies have been pursued for the insertion of antibody genes into the viral genomes toward efficient and/or replication-dependent gene expression and minimizing the required genomic space (right panel). ab, antibody; Env, envelope; HSV, herpes simplex virus; MeV, measles virus; NDV, Newcastle disease virus; ORF, open reading frame; VSV, vesicular stomatitis virus
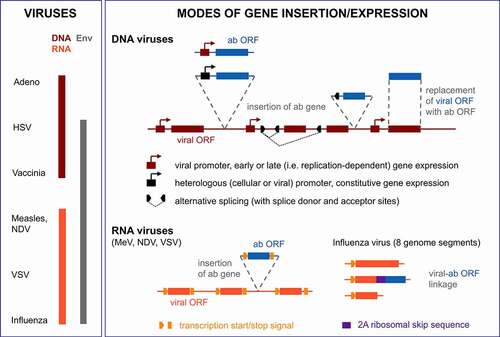
Figure 3. Antibody formats utilized in viro-antibody therapy. BiTE, bispecific T cell engager; CH/CL, constant domain of heavy/light chain; Fab, antigen-binding fragment; Fc, constant fragment of antibody; IgG, immunoglobulin G; RNase, ribonuclease; scFv, single chain variable fragment; sdAb, single domain antibody (variable fragment of camelid antibody heavy chain); VH/VL, variable domain of heavy/light chain. For the scFv fusion proteins, proteins might be fused at the C-terminus of the scFv (cytokine, trimerization peptide and FasL) or at the N-terminus (RNase); scFvs might be in VH-VL or VL-VH configuration
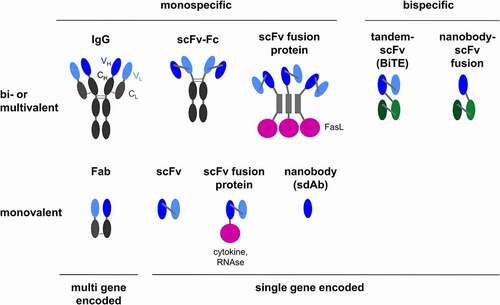
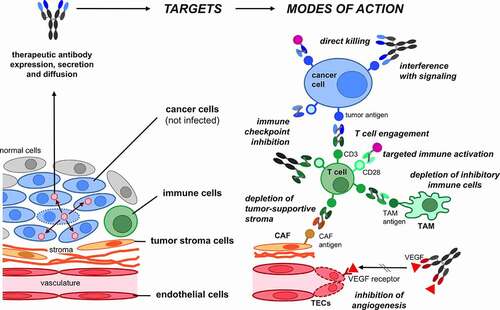
Figure 4. Target cells and modes of action of OV-encoded antibodies. The depicted cancer targets and direct or immune- or stroma-mediated modes of action have been reported. For some of the depicted modes of action only examples of the explored antibody formats are depicted (see for a comprehensive list). CAF, cancer-associated fibroblast; TAM, tumor-associated macrophage; TECs, tumor endothelial cells