Figures & data
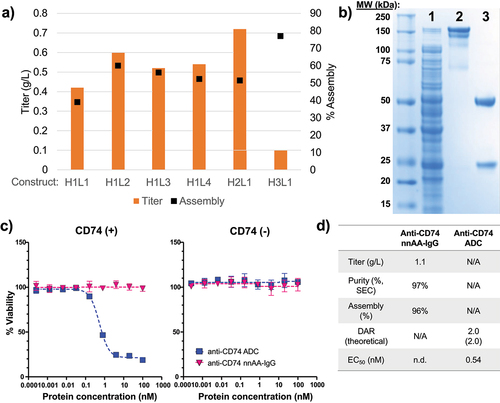
Figure 1. Expression of therapeutically relevant antibody fragments and full-length IgGs in E. coli strain SBDG419.
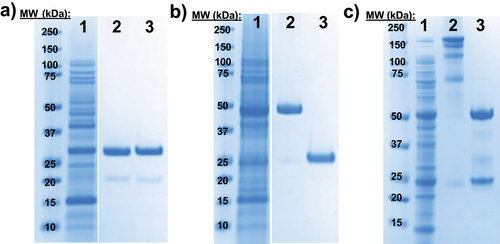
Table 1. Summary of titers and amber suppression efficiency of pAMF incorporation into nnAA-LC and nnAA-IgG produced in E. coli. For titer calculation method descriptions, refer to the materials and methods section within the supplementary information. Amber suppression efficiencies were calculated by dividing the titer (*from bioreactors, **from shake flasks) of nnAA-containing proteins by the titer of the proteins without nnAA incorporation.
Figure 2. Expression and activity of nnAA-LC, nnAA-IgG-X and DAR8 ADC-X.
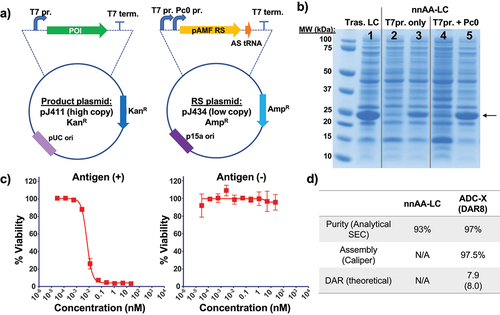
Figure 3. Schematic of cell free protein synthesis-based production of nnAA-IgG-X and ADC-X using nnAA-LC as pre-fabricated light chain.
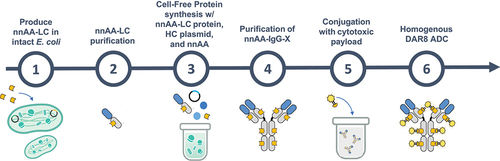
Figure 4. Expression, purification, and biological activity of the anti-CD74 nnAA-IgG and its derivative ADC.