Figures & data
Figure 1. Timeline of regulatory approval of bsAbs with their respective MOA. Linvoseltamab, odronextamab and tarlatamab are currently under regulatory review with a decision anticipated in 2024. Created with Biorender.com.
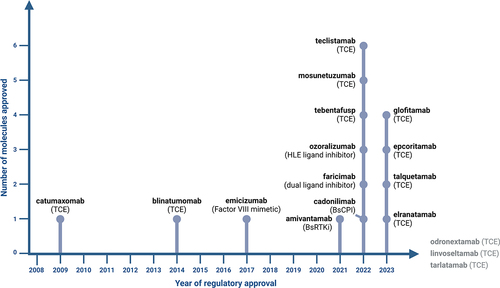
Table 1. Approved bsAbs and bsAbs under regulatory review.
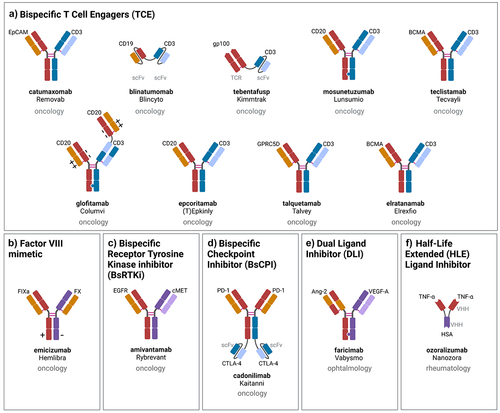
Figure 2. Schematic representation, indication and mechanism of action of approved bsAbs: a) T cell engagers (TCE), b) factor VIII mimetic, dual signaling inhibition: c) bispecific receptor tyrosine kinase (RTK) inhibitor (BsRtki), d) bispecific checkpoint inhibitor (BsCPI), e) dual ligand inhibitor (DLI), f) half-life extended (HLE) ligand inhibitor. Created with Biorender.com.