Figures & data
Figure 1. Determinants of the use of poorly selective antibodies in research.
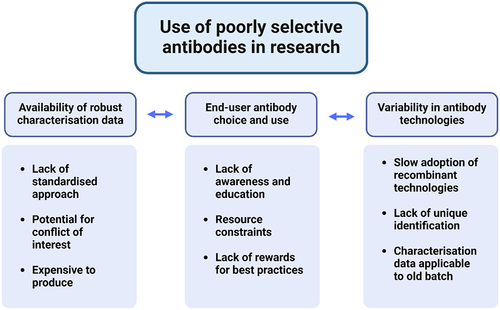
Figure 2. A strategy for improving the use of antibodies in research.
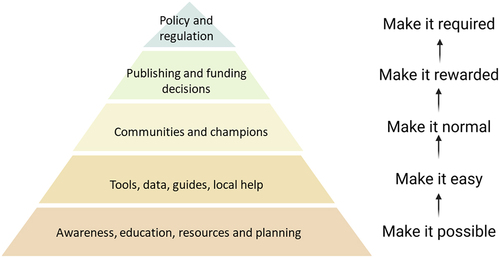
Figure 3. Working with stakeholders across the scientific community to develop consensus solutions.

