Figures & data
Table 1. The six criteria critical for clinical development and commercial manufacturing of a good therapeutic bispecific antibody as highlighted by Nie et al.Citation19.
Figure 1. RUBY format structure. RUBY bsAbs comprise of an IgG (dark green, light green and gray) coupled to two fabs (dark and light turquoise). Three separate chains make up a construct, (1) a long chain that consists of the IgG heavy chain (dark green and gray) and the light chain of the additional fab fragment (light turquoise), (2) a light chain (light green) that binds to the VH and CH1 domains of the IgG part, and (3) a short heavy chain (dark turquoise) that binds to the light chain appended to the IgG.
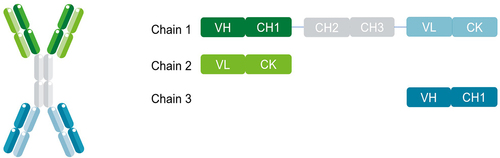
Table 2. Summary of RUBY variant 9 and deimmunizing (in bold) mutations.
Figure 2. Stability characterization of RUBY v9 bsAbs. (a) Ability of the molecules to withstand shear stress determined by measuring A280 before and after rapid agitation. (b) Colloidal stability of the molecules was determined by incubating them at various PEG concentrations. (c) Molecules were incubated in human serum or BSA for seven days after which a dual ELISA was performed. (d) Molecules were incubated at various temperatures for up to two weeks and dual ELISA was performed thereafter. (e) Stability of the molecules in 8 different buffers was analyzed by incubation at 40°C for 4 weeks. Representative results of RUBY v9 #1 after SEC-HPLC analysis are shown for HMWs (f) and LMWs (g). (h) RUBY bsAbs were incubated at pH 3.5 up to 2 hours. SEC-HPLC analysis was performed after neutralization of the proteins at 30, 60, 90 or 120 minutes.
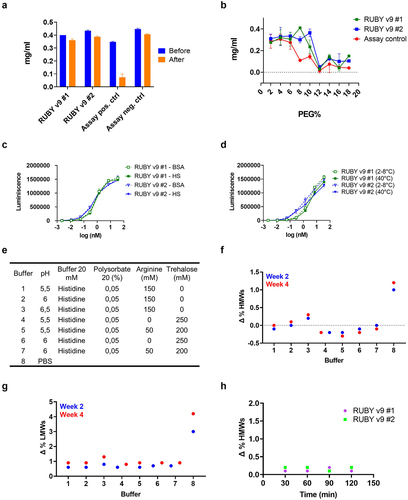
Table 3. Measurements of melting temperatures (Tm) for different RUBYv9 bsAbs.
Table 4. Changes in degradation after different treatments to evaluate the stability of RUBY v9 bsAbs. Protein concentrations ranged between 0.5 and 3 mg/ml in non-optimized buffer (PBS).
Table 5. 1:1 binding kinetics of RUBY bsAbs and corresponding mAb controls.
Figure 3. Assessing binding and in vitro functionality of RUBY v9 bsAbs. (a) Dual ELISA showing simultaneous binding of RUBY v9 bsAbs to their respective antigen targets. ELISA plates were coated with one antigen, RUBY bsAbs were added followed by detection using a biotinylated second antigen. (b) The activity of a CD40-EpCAM RUBY v9 bsAb in a CD40 reporter assay. CD40 reporter cells (Promega) were incubated with EpCAM-transfected CHO cells or wild type CHO cells in the presence of the bsAb. CD40 activation was measured according to the manufacturer’s instructions. (c) Demonstration of the agonistic function of the CD137-5T4 RUBY bsAbs v9 bsAb on human CD8 T cells where a dose response dependent IFN-gamma production (absolute values) by human CD8 T cells from one representative individual donor activated with the bispecific constructs in the presence or absence of immobilized 5T4-Fc was observed. Obtained mean (and SD) IFN-gamma levels from one representative individual donor from experiment set 2 is shown.
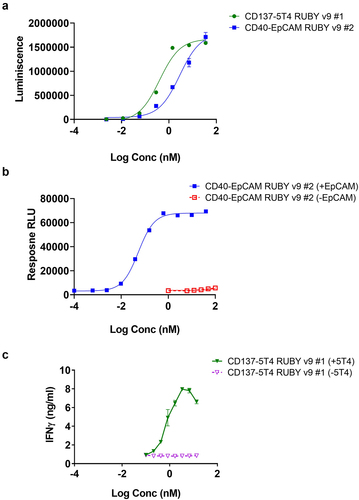
Table 6. Kinetic measurements for RUBY bsAbs of different Fc isotype variants and monoclonal isotype controls after binding to hFcγri, hFcγriia and hFcγriiia 176 V.
Table 7. Kinetic measurements for RUBY bsAbs of different Fc isotype variants and monoclonal isotype controls after binding to mFcγri, mFcγriib, mFcγriii and mFcγr IV.
Figure 4. RUBY bsAbs interaction with human FcRn and pharmacokinetics. BLI binding kinetics measurements of (a) RUBY bsAb and (b) monoclonal antibody control displaying similar interaction with human FcRn. (c) C57bl/6 mice were intravenously (i.v.) administered with molar equivalent doses of a human IgG1 LALA-mutated RUBY v9 bsAb (167 µg) or a corresponding mAb control (100 µg). Blood was collected 1, 2, 4, 24, 72 and 168 hours post dosing (n = 3/timepoint) and serum concentration of human IgG was measured using a human heavy chain-specific ELISA. (d) Human CD40 transgenic mice were administered with molar equivalent doses i.v. of two CD40-TAA RUBY bsAbs (360 µg) and a CD40 mAb (216 µg). Blood was collected 0.5, 1, 2, 4, 6, 24, 48 and 72 hours post dosing (n = 3/timepoint) and serum concentration of human IgG was measured using a human heavy chain-specific ELISA. (e) CEA-CD40 RUBY bsAb #2 was administered i.v. at 10 or 37.5 mg/kg to one female and one male cynomolgus monkey per dose level. Blood was collected 0.08, 0.5, 2, 4, 8, 24, 48, 72, 96 and 168 hours post dosing and serum concentration of human IgG was measured using a human CD40 and human CEACAM5 dual target ELISA. The ratio of full RUBY molecules over total human IgG in the serum of (f) human CD40 transgenic mice following i.V. administration of 417 µg CD40-EpCAM RUBY v9 #2, or (g) cynomolgus monkeys administered 10–37.5 mg/kg CEA-CD40 RUBY bsAb #2 was determined using ELISA. The graphs show the mean (±SD) of 3 mice/timepoint (c, c, and f), measured values over time for individual animals (e) or the mean (±SD) of four animals (g).
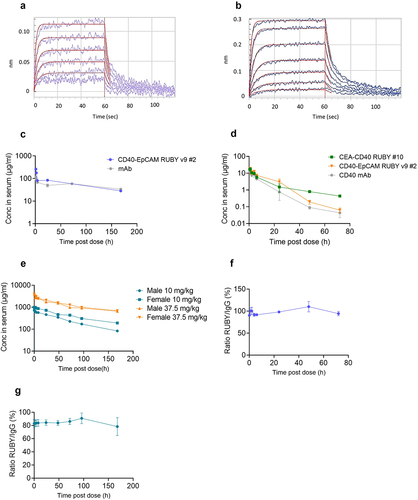
Table 8. Kinetic measurements of controls and RUBY bsAbs of different fc isotypes binding to FcRn receptors of both human and mouse origin.
Figure 5. Tumor localization and hydrodynamic sizes of RUBY bsAbs and controls. (a) RUBY v9 bsAb efficiently localized to the tumor area, where they bound EpCAM expressing tumor. Mice were inoculated s.c. with MB49 tumors expressing human EpCAM or the same cell line lacking the EpCAM expression. The mice received a single CD40-EpCAM RUBY v9 #2 bsAb, CD40 mAb or vehicle i.p. injection and 24 hours later the tumors were collected and the frequency of IgG+ cells were analyzed by flow cytometry. (b) A RUBY bsAb and (c) Monoclonal antibody control displayed similar hydrodynamic sizes as determined by DLS.
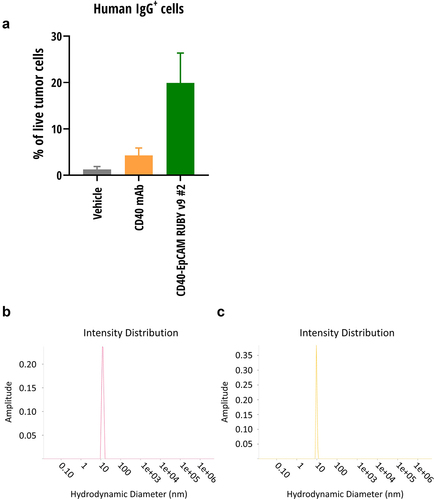
Table 9. Hydrodynamic diameter of monomeric fraction of RUBY bsAbs and control mAb measured by DLS.
