Figures & data
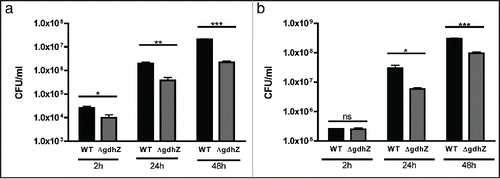
Figure 1. Metabolic control of cell division by GdhZ in Caulobacter crescentus C. crescentus has developed a complex asymmetric cell cycle to optimize its survival in oligotrophic environments. (a) Each cell division produces a small swarmer cell and a large stalked cell. The swarmer cell is proposed to be a settler looking for new environments whereas the stalked cell is responsible for colonizing these environments by giving birth to progeny cells. When a swarmer cell finds favorable conditions, it differentiates into a stalk cell to enter into a replicative cycle. (b-c) C. crescentus has evolved a system in which the NAD-dependent glutamate dehydrogenase GdhZ acts as a proxy, signaling nutrient availability to the division apparatus, thereby coordinating growth with cell division. FtsZ and GdhZ are respectively represented in green and red.Citation5
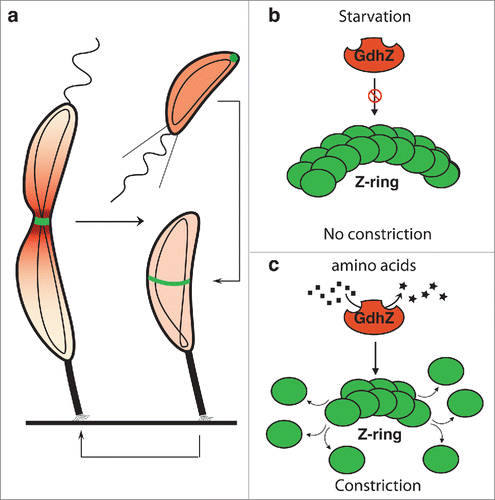
Figure 2. GdhZ coordinates growth with cell division in Brucella abortus. (a) Growth of B. abortus wild-type (black) and ΔgdhZBa (gray) cells in complex medium (2YT) or in synthetic medium with xylose as the only carbon source (Plommet Xylose), showing GdhZ is required for optimal growth in complex mediium. (b) Phase contrast imaging of B. abortus wild-type and ΔgdhZBa cells grown in complex medium (2YT) illustrating the morphological defects with elongated and branched cells (arrows) developed by ΔgdhZBa cells. Cells were imaged in mid-exponential phase of growth. Scale bar, 2 μm.
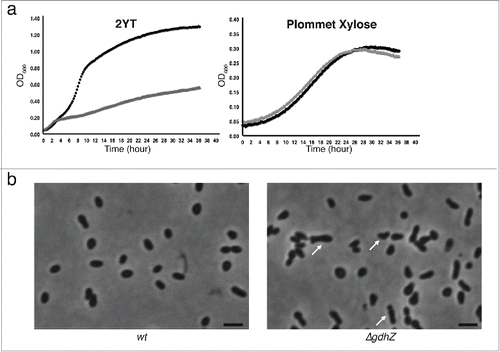
Figure 3. GdhZ is required for efficient replication of Brucella abortus inside murine raw macrophages. Internalization and intracellular replication of B. abortus wild-type and ΔgdhZBa cells into murine raw macrophages. RAW 264.7 macrophages were cultured at 37 °C (5 % CO2 atmosphere) in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Gibco), 4.5 g/L glucose, 1.5 g/L NaHCO3 and 4 mM glutamine. Cultures of Brucella were prepared in DMEM at a multiplicity of infection of 50. Bacteria were centrifuged at 400 x g for 10 min at 4 °C and then incubated for 1 hr at 37 °C (5 % CO2 atmosphere). Cells were washed twice with fresh medium and then incubated in medium supplemented with 50 μg/ml gentamicin to kill extracellular bacteria. Prior to infections, bacteria were grown in (a) 2YT or (b) 2YT + 0.2 % glucose. The significant pairwise comparisons are indicated for p < 0.5 (*), p < 0.01 (**) and p < 0.001 (***) in Student t-tests.