Figures & data
Figure 1. Main discoveries in extracellular vesicle biology. Timeline showing the main discoveries in the extracellular vesicle research.
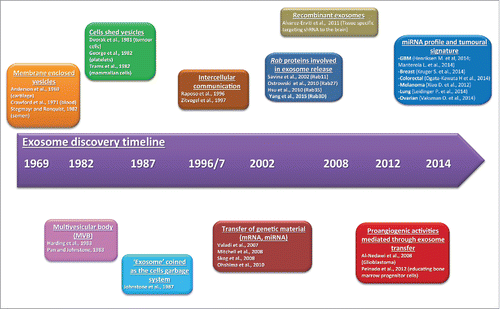
Figure 2. Role of sortilin in EV biogenesis. Sortilin is initially synthesized in the constitutive secretory pathway as a precursor encoding a short propeptide sequence. The propeptide is cleaved by pro-protein convertases at the TGN allowing sortilin to enter the secretory pathway (stage 1). There are a number of likely routes that sortilin can be trafficked. Sortilin can be trafficked along a number of possible routes such as trafficking to the plasma membrane through constitutive secretory vesicles (stage 2). Alternatively, sortilin could be anterograde transported from the TGN by itself or with its binding partners to the early endosomes (stage 3). Sortilin present at the cell surface or in the endocytic pathway could be cleaved by disintegrin and metalloproteinase domain-converting protein (ADAM) 10 or ADAM17, and followed by g-secretase (stage 5). Following endoproteolytic cleavage, sortilin could form a heterotrimeric complex with TrkB and EGFR (TES complex) which is internalized through a clathrin-dependent endocytosis process into early endosomes (stage 6). At the plasma membrane, the purple spots represent clathrin associated with vesicles (clathrin-coated vesicles [CCV]) or the bilayered clathrin coats at the endosome. The intraluminal vesicles (ILV) are formed by an invagination event at the membrane of the late endosomes/multivesicular body (MVB). Sortilin may play a role in the recruitment of certain cargo such as its binding partners- TrkB and EGFR, which could be an ESCRT-dependent mechanism. The MVB and its content could be degraded via the lysosome-mediated pathway for degradation or alternatively the MVB are transported to the cell surface were they dock at the plasma membrane requiring Rab27A to release the vesicles into the extracellular space (stage 7). The exosomes carrying the TES complex could be released and taken up in the target cell. The uptake of TES-containing exosomes initiates cellular communication through upregulation of cell signaling events by the induction of cell survival through the EGFR cascade and the angiogenesis process (stage 8).
![Figure 2. Role of sortilin in EV biogenesis. Sortilin is initially synthesized in the constitutive secretory pathway as a precursor encoding a short propeptide sequence. The propeptide is cleaved by pro-protein convertases at the TGN allowing sortilin to enter the secretory pathway (stage 1). There are a number of likely routes that sortilin can be trafficked. Sortilin can be trafficked along a number of possible routes such as trafficking to the plasma membrane through constitutive secretory vesicles (stage 2). Alternatively, sortilin could be anterograde transported from the TGN by itself or with its binding partners to the early endosomes (stage 3). Sortilin present at the cell surface or in the endocytic pathway could be cleaved by disintegrin and metalloproteinase domain-converting protein (ADAM) 10 or ADAM17, and followed by g-secretase (stage 5). Following endoproteolytic cleavage, sortilin could form a heterotrimeric complex with TrkB and EGFR (TES complex) which is internalized through a clathrin-dependent endocytosis process into early endosomes (stage 6). At the plasma membrane, the purple spots represent clathrin associated with vesicles (clathrin-coated vesicles [CCV]) or the bilayered clathrin coats at the endosome. The intraluminal vesicles (ILV) are formed by an invagination event at the membrane of the late endosomes/multivesicular body (MVB). Sortilin may play a role in the recruitment of certain cargo such as its binding partners- TrkB and EGFR, which could be an ESCRT-dependent mechanism. The MVB and its content could be degraded via the lysosome-mediated pathway for degradation or alternatively the MVB are transported to the cell surface were they dock at the plasma membrane requiring Rab27A to release the vesicles into the extracellular space (stage 7). The exosomes carrying the TES complex could be released and taken up in the target cell. The uptake of TES-containing exosomes initiates cellular communication through upregulation of cell signaling events by the induction of cell survival through the EGFR cascade and the angiogenesis process (stage 8).](/cms/asset/736573c4-1bbd-48ce-b63e-92c465bab136/kcib_a_1130192_f0002_c.gif)
