Figures & data
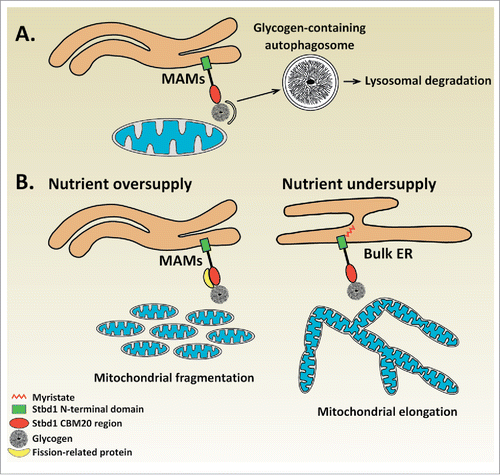
Figure 1. Proposed role for Stbd1-mediated recruitment of glycogen to ER-mitochondria contact sites. (A) Glycogen autophagy (Glycophagy) | Binding of glycogen to non-myristoylated Stbd1 promotes its targeting to ER-mitochondria contact sites. The recruitment of glycogen to ER-mitochondria junctions which have been identified as the sites of autophagosome formation could serve its sequestration into autophagosomes for lysosomal degradation. (B) Nutrient sensing mechanism | Stbd1-mediated recruitment of glycogen to ER-mitochondria contacts may represent a mechanism for nutrient sensing that influences mitochondrial morphology. Upon nutrient oversupply, intracellular glycogen levels increase leading to the targeting of glycogen-bound, non-myristoylated Stbd1 to ER-mitochondria contacts. Stbd1 may interact at ER-mitochondria contact sites with proteins involved in mitochondrial fission such as Drp1 or its known adaptors resulting in the fragmentation of the mitochondrial network. On the other hand, under conditions of nutrient undersupply, cellular glycogen is degraded and hence Stbd1 is preferentially targeted to bulk ER instead of ER-mitochondria contact sites. Failure of recruiting mitochondrial fission proteins through Stbd1 at ER-mitochondria interfaces would consequently result in the formation of fused, elongated mitochondria. This model is in accordance with the observed effects of Stbd1 on mitochondrial morphology, i.e. overexpression of non-myristoylated Stbd1 causes fragmentation whereas Stbd1 knockdown elongation of the mitochondrial network, and the available data on the correlation between nutrient availability and morphology of the mitochondrial network.