Figures & data
Figure 1. Formal scheme showing proton exchanges in Rubisco catalysis. (a) Chemical H exchanges during catalytic processes. The starting substrate is protiated ribulose 1,5-bisphosphate (denoted as h-RuBP). Enolization reversibility can generate deuterated RuBP, with a 2H atom at C3 (d-RuBP) or both C3 and O3 (d2-RuBP) via one or two solvent exchange steps. Solvent heavy water molecules involved in different steps of catalysis are labeled with different colors to allow tracing the fate of deuterons (2H+). For clarity, only one residue is shown in this figure, Lys 201 (numbering in spinach), which is responsible for proton abstraction in enolization in its carbamylated form. Note that the C3 proton abstracted during enolization is eventually lost during catalysis and reprotonation uses a solvent water molecule, thus forming a 3-phosphoglycerate (PGA) molecule (referred to as “upper”) deuterated at C2. In this panel, hydroxyl groups are simply represented as O–H for simplicity while in the chemical mechanism, H atoms can be shared with basic groups of active site residues, forming a partially charged oxygen atom. Note that the H (2H) atom at O2 in PGA can be slowly exchanged with solvent water after having been released by the enzyme (discussed in main text). (b) Summary of H exchange history of protons in RuBP.
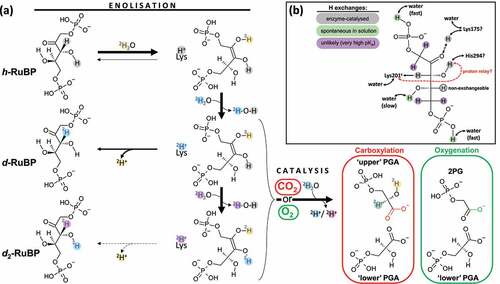
Figure 2. RuBP isotopologues observed during Rubisco-catalyzed carboxylation, monitored using high resolution (exact mass) LC-MS. Substrate ribulose 1,5-bisphosphate (RuBP) was natural (protiated RuBP) and the solvent was heavy water (2H2O). Here, signals associated with the major ion are shown, i.e., [M–2H]2 – with a monoisotopic m/z value of 153.984921 a.m.u. Units of the x-axis are in half a.m.u. because of the charge (–2) of the ion of interest. (a) Kinetics of protiated 12C (monoisotopic, denoted as m) and protiated 13C RuBP disappearance and formation of deuterated RuBP (2H) during the reaction (assay of 60 seconds with CO2 equilibrated at 100 ppm in the gas phase). The inset shows the appearance of 12C dideuterated and 13C monodeuterated RuBP. (b) Isotopologue composition at 60s when CO2 is equilibrated at 100, 400 or 800 ppm in the gas phase. In all cases, the initial amount of RuBP was adjusted to reach ≈40% of initial concentration at the end of the assay. Note the much higher content in deuterated RuBP at low (100 ppm) and moderate (400 ppm) CO2 concentration, compared to high CO2 (800 ppm). As in (a), the inset shows 12C dideuterated and 13C monodeuterated isotopologues.
![Figure 2. RuBP isotopologues observed during Rubisco-catalyzed carboxylation, monitored using high resolution (exact mass) LC-MS. Substrate ribulose 1,5-bisphosphate (RuBP) was natural (protiated RuBP) and the solvent was heavy water (2H2O). Here, signals associated with the major ion are shown, i.e., [M–2H]2 – with a monoisotopic m/z value of 153.984921 a.m.u. Units of the x-axis are in half a.m.u. because of the charge (–2) of the ion of interest. (a) Kinetics of protiated 12C (monoisotopic, denoted as m) and protiated 13C RuBP disappearance and formation of deuterated RuBP (2H) during the reaction (assay of 60 seconds with CO2 equilibrated at 100 ppm in the gas phase). The inset shows the appearance of 12C dideuterated and 13C monodeuterated RuBP. (b) Isotopologue composition at 60s when CO2 is equilibrated at 100, 400 or 800 ppm in the gas phase. In all cases, the initial amount of RuBP was adjusted to reach ≈40% of initial concentration at the end of the assay. Note the much higher content in deuterated RuBP at low (100 ppm) and moderate (400 ppm) CO2 concentration, compared to high CO2 (800 ppm). As in (a), the inset shows 12C dideuterated and 13C monodeuterated isotopologues.](/cms/asset/f5a294f9-305b-4ad8-bdb0-7799270219c7/kcib_a_2039431_f0002_oc.jpg)
Figure 3. Isotopologues of products of Rubisco catalysis: oxygenation (a,b) and carboxylation (c,d), monitored using high-resolution (exact mass) LC-MS analysis. Substrate ribulose 1,5-bisphosphate (RuBP) was natural (protiated RuBP) and the solvent was heavy water (2H2O). Major ions of 2-phosphoglycolate and 3-phosphoglycerate are (monoisotopic): [M–H] – at 154.9745 a.m.u. and [M–H] – at 184.9851 a.m.u., respectively. As in , the figure shows the monoisotopic species (m), 13C and 2H isotopologues (a,b,c) and double isotopologues (inset of b, and d). Results presented here are associated with a 60s-assay (a,b,c) or the effect of CO2 concentration (d) equilibrated with 100, 400 or 800 ppm in the gas phase. In (d), the asterisk (*) stands for other features (m/z) present in the assay and unrelated to 2-phosphoglycolate or 3-phosphoglycerate. In (a) and (b), no dideuterated 3-phosphoglycerate or 2-phosphoglycolate could be observed.
![Figure 3. Isotopologues of products of Rubisco catalysis: oxygenation (a,b) and carboxylation (c,d), monitored using high-resolution (exact mass) LC-MS analysis. Substrate ribulose 1,5-bisphosphate (RuBP) was natural (protiated RuBP) and the solvent was heavy water (2H2O). Major ions of 2-phosphoglycolate and 3-phosphoglycerate are (monoisotopic): [M–H] – at 154.9745 a.m.u. and [M–H] – at 184.9851 a.m.u., respectively. As in Figure 2, the figure shows the monoisotopic species (m), 13C and 2H isotopologues (a,b,c) and double isotopologues (inset of b, and d). Results presented here are associated with a 60s-assay (a,b,c) or the effect of CO2 concentration (d) equilibrated with 100, 400 or 800 ppm in the gas phase. In (d), the asterisk (*) stands for other features (m/z) present in the assay and unrelated to 2-phosphoglycolate or 3-phosphoglycerate. In (a) and (b), no dideuterated 3-phosphoglycerate or 2-phosphoglycolate could be observed.](/cms/asset/3e201e1a-cb19-43b9-a2b8-7ad7e5ec7ef3/kcib_a_2039431_f0003_oc.jpg)
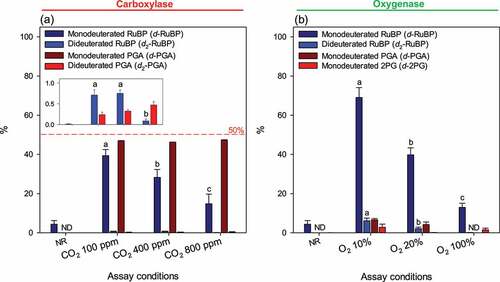
Figure 4. Abundance of deuterated isotopologues in substrate and products of Rubisco catalysis. Substrate ribulose 1,5-bisphosphate (RuBP) was natural (protiated RuBP) and the solvent was heavy water (2H2O). RuBP and 3-phosphoglycerate (PGA) after 60s of carboxylase assay (a), RuBP, PGA and 2-phopshoglycerate (2PG) after 60s of oxygenase assay (b). The inset in (a) is a magnification to facilitate reading the percentage of dideuterated species. The value shown here is the percentage (%) with respect to the total pool size of the metabolite of interest; for example, 40% d-RuBP means than monodeuterated RuBP represents 40% of total RuBP (total = non-deuterated + monodeuterated + dideuterated + other isotopic forms such as the 13C isotopologue). Data shown are mean ± SE (n = 3). Gases concentrations are shown with respect to the gas phase used to equilibrate the reacting medium. Letters stand for statistical classes (P< 0.05). ND, not detected. NR, no reaction.