Figures & data
Figure 1. (colour online) Overview of the authorisation procedure for feed additives as outlined by Regulation (EC) No. 1831/2003, showing the interactions between the applicants and the European Union Reference Laboratory (EURL), the European Food Safety Authority (EFSA) and the European Commission. The main deliverables of the organisations involved are the EURL evaluation report of the analytical method, the scientific opinion of EFSA, and the regulation issued by the European Commission to grant or deny authorisation of the feed additive.
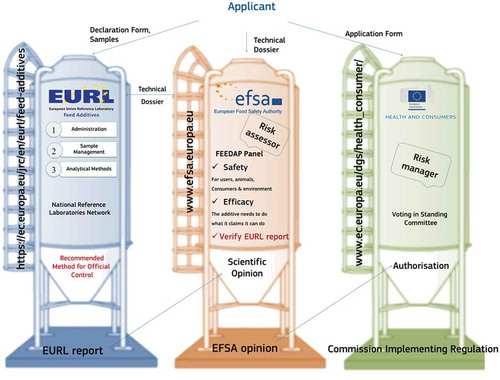
Table 1. Example for the authorisation of the feed additive ‘Deccox’ containing the coccidiostat decoquinate as active substance.
Table 2. EURL evaluation reports on analytical methods in the period 2005–14: number of feed additives covered by these reports and distribution across categories and functional groups.
Figure 2. (colour online) Evaluation reports issued by the EURL-FA and their impact expressed in terms of number of EFSA opinions and European Commission regulations delivered per year and total number across all years.
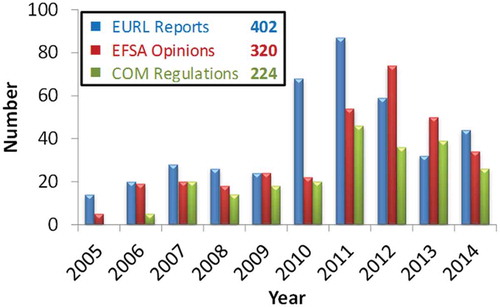
Table 3. Authorisation of feed additives reducing the mycotoxin content in feed.
