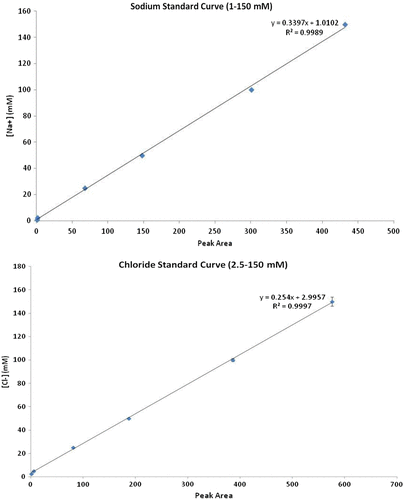
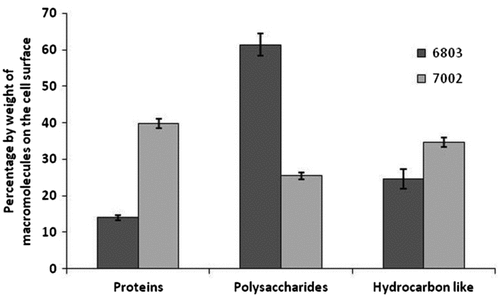
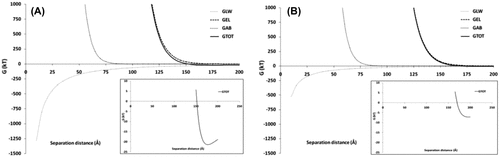
Figures & data
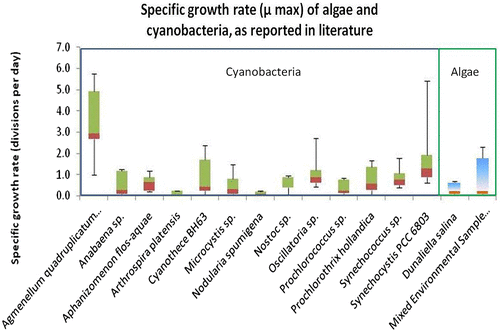
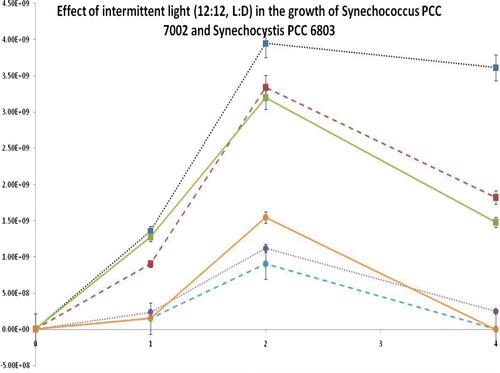
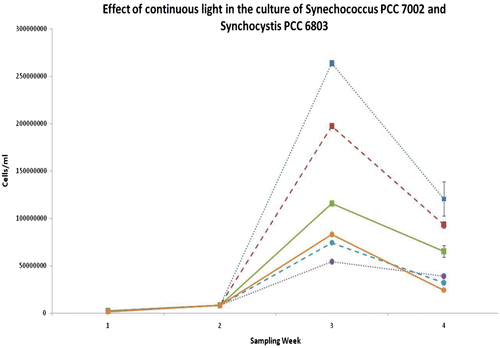
Table 1 Divisions per day as calculated by flow cytometry. The table below demonstrates growth rate (per day) for Synechococcus PCC 7002 and Synechocystis PCC 6803, under different illuminations. Red indicates highest growth observed for each organism over 3 weeks of culture. Green indicates lowest growth for each organism per period.