Figures & data
Fig. 1. Schematic presentation of installation setup for the synthesis of C–TiO2 [Citation21].
Notes: (1) Gas cylinder with argon, (2) controller, (3) Dreschel bottle with ethanol, (4) pipe furnace, (5) combustion boat with powder sample, and (6) scrubber.
![Fig. 1. Schematic presentation of installation setup for the synthesis of C–TiO2 [Citation21].Notes: (1) Gas cylinder with argon, (2) controller, (3) Dreschel bottle with ethanol, (4) pipe furnace, (5) combustion boat with powder sample, and (6) scrubber.](/cms/asset/e37d8c12-da6f-420c-ae45-0c4fa30e1d61/tdwt_a_1094678_f0001_oc.gif)
Fig. 2. SEM of (a) unmodified TiO2, (b) C–TiO2-200, (c) C–TiO2-300, and (d) C–TiO2-400. The scale bar represents 500 nm.
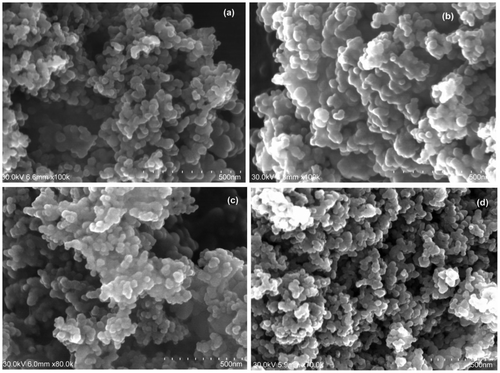
Fig. 3. Nitrogen adsorption/desorption isotherm of (a) TiO2, (b) C–TiO2-200, (c) C–TiO2-300, and (d) C–TiO2-400.
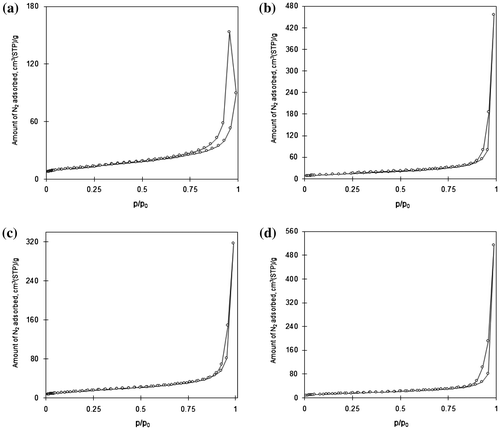
Table 1 Textual properties of TiO2 and C–TiO2 adsorbents
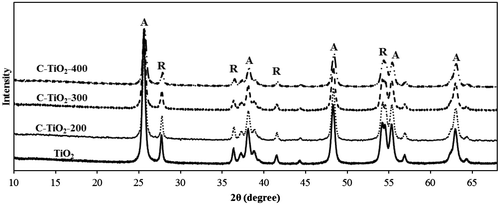
Fig. 5. FTIR spectra of modified and unmodified TiO2, (a) C–TiO2-200, (b) C–TiO2-300, (c) C–TiO2-400, and (d) TiO2.
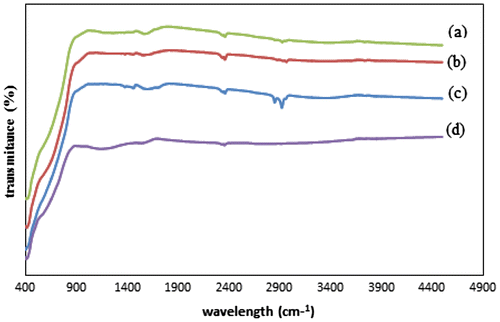
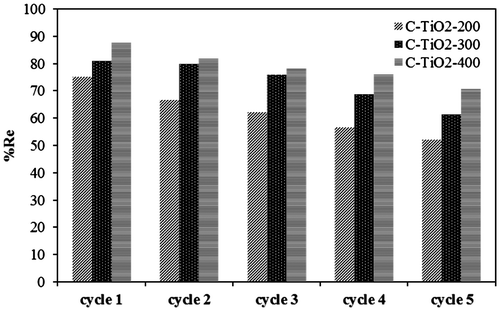
Fig. 6. Time dependency of the adsorption of MO by C–TiO2 adsorbents. Solid and dashed lines represent the predicted qt values by PSO model.
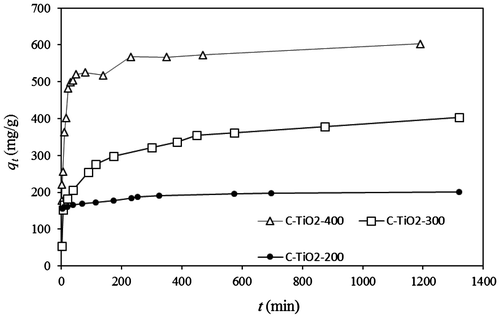
Table 2 Obtained constants of PFO and PSO rate equations for the adsorption of MO onto C–TiO2 adsorbents
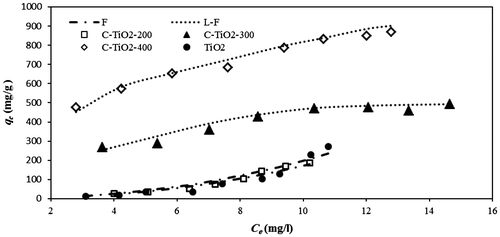
Table 3 Obtained isotherm constants for adsorption of MO onto TiO2 and C–TiO2 adsorbents
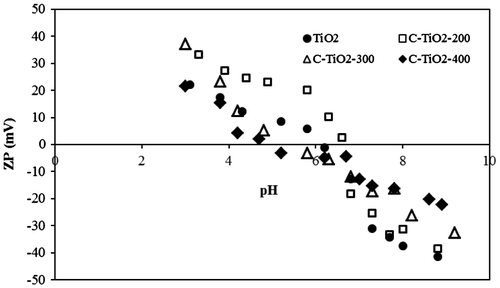
Fig. 8. Removal percentage of MO by (a) C–TiO2-200 (b) C–TiO2-300 and (c) C–TiO2-400 at different pH.
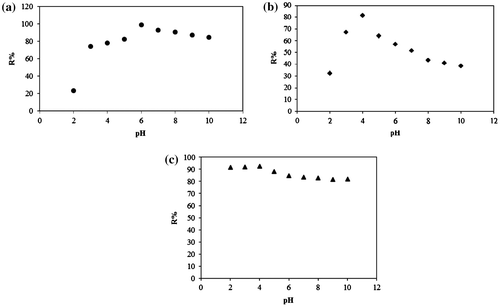
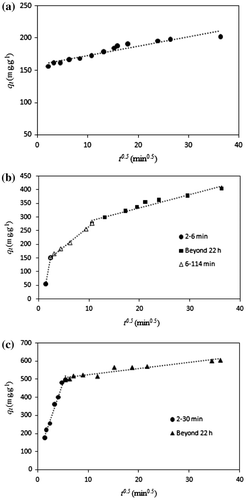
Fig. 12. The intraparticle diffusion plot for adsorption of MO onto (a) C–TiO2-200, (b) C–TiO2-300 and (c) C–TiO2-400.